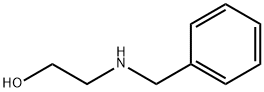
N-Benzylethanolamine synthesis
- Product Name:N-Benzylethanolamine
- CAS Number:104-63-2
- Molecular formula:C9H13NO
- Molecular Weight:151.21
Yield:104-63-2 100%
Reaction Conditions:
Stage #1:benzaldehyde;ethanolamine in methanol at 20; for 0.25 h;
Stage #2: with methanol;sodium tetrahydroborate at 0 - 20;
Steps:
5; 128
General procedures:; Scheme 5; To a solution of an aldehyde (1 equiv) in methanol was added an amine derivative (1 equiv). After stirring at room temperature for 15 min, the solution was cooled to 0 0C prior to the addition of sodium borohydride (0.5 equiv) portion wise. The resulting solution was stirred at room temperature for 1 h. After the addition of water (3 mL), methanol was removed under reduced pressure and the resulting aqueous phase was extracted with DCM. The combined extracts were dried over Na2SO4 and concentrated in vacuo to give 5-1 in quantitative yield. 5-1 (1 equiv) was added to the mixture of 4-bromophenyl isocyanate (1 equiv) in dry DCM (3 mL) at 0 0C. After stirring at room temperature for 0.5 h, the solvent was removed to give urea 5-2 in quantitative yield. The urea derivative 5-2 (1 equiv) and the boronic acid ester (1.5 equiv) were dissolved in degassed 5: 1 dioxane/H2O. Pd(PPh3)4 (0.1 equiv) and 2M solution Of K2CO3 (3 equiv) were then added sequentially and the mixture was heated at 95 0C for 4h. After cooling to room temperature, the mixture was diluted with water and extracted with ethyl acetate. The combined organic extracts were washed with saturated brine, dried over Na2SO4, concentrated in vacuo to give crude 5-3. This crude material was subjected to preparative HPLC to give 5-3 as a white solid.; Example 128. 3-(4-ϖH-pyrazol-4-v0phenyl)-l -benzyl- l-(2-hvdroxyethyl)urea; Procedures in Scheme 5 were utilized to synthesize this compound. LC/MS: Ci9H20N4O2 (M+l) 337. Single peak at both 215 nm and 254 nm in analytical HPLC traces.
References:
FENG, Yangbo;LOGRASSO, Philip;SCHROETER, Thomas;YIN, Yan WO2010/36316, 2010, A1 Location in patent:Page/Page column 76-77
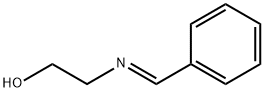
124948-51-2
4 suppliers
inquiry

104-63-2
361 suppliers
$7.00/10g
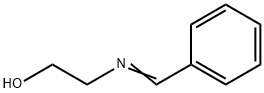
770-37-6
6 suppliers
inquiry

104-63-2
361 suppliers
$7.00/10g

141-43-5
920 suppliers
$9.00/10g

100-51-6
1433 suppliers
$5.00/100g

104-63-2
361 suppliers
$7.00/10g
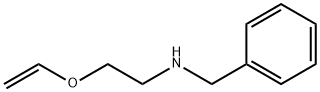
73731-97-2
15 suppliers
$378.00/1g

104-63-2
361 suppliers
$7.00/10g
