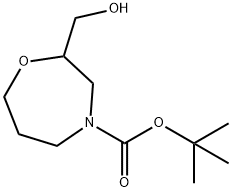
4-Boc-2-(hydroxymethyl)homomorpholine synthesis
- Product Name:4-Boc-2-(hydroxymethyl)homomorpholine
- CAS Number:1174020-52-0
- Molecular formula:C11H21NO4
- Molecular Weight:231.29

24424-99-5
810 suppliers
$13.50/25G
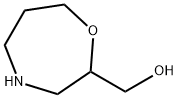
1207254-23-6
26 suppliers
$45.00/10mg

1174020-52-0
34 suppliers
$53.00/250mg
Yield: 82.6%
Reaction Conditions:
Stage #1:di-tert-butyl dicarbonate;[1,4]Oxazepan-2-yl-methanol with triethylamine in dichloromethane at 0 - 20;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water
Steps:
106.4
Step-4: 2-Hydroxymethyl-[1,4]oxazepane-4-carboxylic acid tert-butyl ester To a solution of [1,4]Oxazepan-2-yl-methanol (550 mg, 4.2 mmol) in DCM was added triethylamine (1.2 ml, 8.4 mmol) and (BOC)2O (1.1 eq, 1 ml, 4.61 mmol) at 0° C. was added and stirred the solution at room temperature for overnight. It was quenched by aq. NaHCO3 solution and extracted with ethyl acetate. The organic layer was then washed with water and brine. Then it was dried over Na2SO4 and concentrated to produce (800 mg, 82.6%) of 2-Hydroxymethyl-[1,4]oxazepane-4-carboxylic acid tert-butyl ester. 1H NMR (400 MHz, DMSO-d6): 4.68 (m, 1H), 3.95 (m, 1H), 3.70 (m, 1H), 3.54-2.98 (m, 6H), 1.74 (m, 2H), 1.46 (m, 1H), 1.39 (s, 4H).
References:
KHAMRAI, Uttam;Karak, Sumit Kumar;Ronsheim, Matthew;Saha, Ashis Kumar US2010/168080, 2010, A1 Location in patent:Page/Page column 89
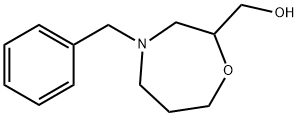
1031442-66-6
25 suppliers
$65.00/100mg

1174020-52-0
34 suppliers
$53.00/250mg
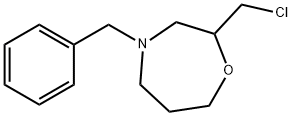
129482-45-7
35 suppliers
inquiry

1174020-52-0
34 suppliers
$53.00/250mg