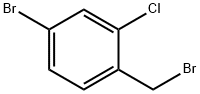
4-BROMO-1-BROMOMETHYL-2-CHLORO-BENZENE synthesis
- Product Name:4-BROMO-1-BROMOMETHYL-2-CHLORO-BENZENE
- CAS Number:89720-77-4
- Molecular formula:C7H5Br2Cl
- Molecular Weight:284.38
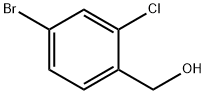
185315-48-4

89720-77-4
To a solution of (4-bromo-2-chlorophenyl)methanol (2.80 g, 12.67 mmol) in dichloromethane (60.0 mL) was added sequentially carbon tetrabromide (4.41 g, 13.30 mmol) and triphenylphosphine (3.48 g, 13.30 mmol) under argon protection and at 0 °C. The reaction mixture was stirred at room temperature for 4 hours. After completion of the reaction, the mixture was concentrated under reduced pressure and the resulting crude product was purified by fast column chromatography (eluent: hexane solution of 10% ethyl acetate) to afford 2-chloro-4-bromobenzyl bromide as a colorless oil (3.59 g, 100% yield).1H NMR (400 MHz, CDCl3): δ 4.53 (s, 2H), 7.29 (d, J = 8.2 Hz. 1H), 7.39 (dd, J = 8.2, 1.9 Hz, 1H), 7.56 (d, J = 1.9 Hz, 1H).
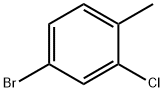
89794-02-5
243 suppliers
$6.00/1g

89720-77-4
82 suppliers
$40.52/1g
Yield: 80%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane for 18 h;Heating / reflux;
Steps:
17
To a solution of 2-Chloro 4-bromo toluene (5.0 g, 24.0 mmol) in carbon tetrachloride is added N-bromo succinimide (4.42 g, 24.8 mmol) and benzoyl peroxide (600 mg, 2.5 mmol) and the reaction is heated at reflux for 18 h. The reaction is allowed to cool to ambient temperature and is extracted saturated Na2COs solution and brine (10 ml each). The organic layer is separated and concentrated in vacuo. The residue is purified by column chromatography eluting with 100% hexanes resulting in 4-Bromo- l-bromomethyl-2-chloro-benzene, as a colorless oil (5.5 g, 80%).
References:
NOVARTIS AG;NOVARTIS PHARMA GMBH WO2007/38459, 2007, A2 Location in patent:Page/Page column 73

185315-48-4
62 suppliers
$55.00/1g

89720-77-4
82 suppliers
$40.52/1g

185315-48-4
62 suppliers
$55.00/1g
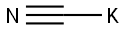
151-50-8
0 suppliers
$36.39/100g

89720-77-4
82 suppliers
$40.52/1g
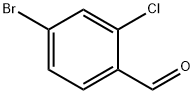
158435-41-7
190 suppliers
$10.00/1 g

89720-77-4
82 suppliers
$40.52/1g
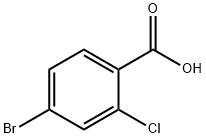
59748-90-2
297 suppliers
$8.00/1g

89720-77-4
82 suppliers
$40.52/1g