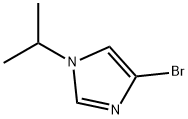
4-BroMo-1-(iso-propyl)-1H-iMidazole synthesis
- Product Name:4-BroMo-1-(iso-propyl)-1H-iMidazole
- CAS Number:623577-60-6
- Molecular formula:C6H9BrN2
- Molecular Weight:189.05
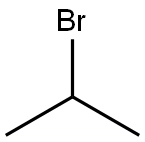
75-26-3

623577-60-6
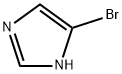
General procedure for the synthesis of 4-bromo-1-isopropyl-1H-imidazole from 2-bromopropane: 4-bromo-1H-imidazole (1.0 g, 6.80 mmol) was dissolved in dimethylformamide (5 mL) at 0 °C and sodium hydride (60% oil suspension, 326 mg, 8.20 mmol) was added with stirring. The reaction mixture was gradually warmed to room temperature and stirred continuously for 30 min. Subsequently, 2-bromopropane (0.70 mL, 7.48 mmol) was added slowly and dropwise. The reaction mixture was stirred at room temperature for 15 hours under nitrogen protection. Upon completion of the reaction, the reaction was quenched with water (10 mL) and extracted with ethyl acetate (3 x 15 mL). The organic phases were combined, washed with water (20 mL) and extracted with 1 M hydrochloric acid (3 x 20 mL). The acidic aqueous phases were combined, washed with ethyl acetate (20 mL) and the pH was adjusted to 12 with ammonium hydroxide, followed by extraction with ethyl acetate (3 × 20 mL). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated. The target product, 4-bromo-1-isopropyl-1H-imidazole, was obtained as a light brown oil (380 mg, 30% yield) by preparative HPLC using an ethyl acetate-isohexane gradient elution.1H NMR (400 MHz, CDCl3) δ: 1.47 (6H, d, J = 6.8 Hz), 4.28-4.32 (1H, m), 6.92 ( 1H, d, J = 1.5 Hz), 7.40 (1H, d, J = 1.5 Hz).MS (ES+) m/z: 189, 191 ([MH]+).

75-26-3
472 suppliers
$10.00/10g

623577-60-6
43 suppliers
$45.00/10mg
Yield:623577-60-6 30%
Reaction Conditions:
Stage #1: 4-bromo-1 H-imidazolewith sodium hydride in N,N-dimethyl-formamide at 0 - 20; for 0.5 h;
Stage #2: isopropyl bromide in N,N-dimethyl-formamide at 20; for 15 h;
Steps:
84.1
A mixture of 4-bromo-1H-imidazole (1.0 g, 6.80 mmol) in dimethylformamide (5 mL) was stirred at 0°C, to which was added sodium hydride, 60% suspension in oil, (326 mg, 8.20 mmol). The reaction mixture was warmed to room temperature and stirred for 30 minutes, followed by dropwise addition of 2-bromopropane (0.70 mL, 7.48 mmol). The reaction mixture was stirred at room temperature for 15 hours under nitrogen, then quenched with water (10 mL) and extracted with ethyl acetate (3 x 15 mL). The combined organic extracts were washed with water (20 mL) and extracted with 1M hydrochloric acid (3 x 20 mL). The combined acidic extracts were washed with ethyl acetate (20 mL), then basified with ammonium hydroxide (pH 12), and extracted with ethyl acetate (3 x 20 mL). The combined organic extracts were dried (Na2SO4), filtered and concentrated. Preparative HPLC, eluting with an ethyl acetate- isohexane gradient, gave the product as a pale brown oil (380 mg, 30%) 8 (1H, 400MHz, CDCl3) 1.47 (6H, d, J = 6.8Hz), 4.28-4. 32 (1H, m), 6.92 (1H, d, J = 1.5 Hz), 7.40 (1H, d, J = 1.5 Hz). MS (ES+) 189,191 ([MH]+).
References:
WO2003/93252,2003,A1 Location in patent:Page/Page column 117
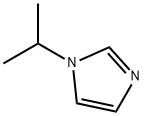
4532-96-1
234 suppliers
$6.00/10g

623577-60-6
43 suppliers
$45.00/10mg

1378632-40-6
18 suppliers
inquiry
2302-25-2
255 suppliers
$6.00/1g

75-26-3
472 suppliers
$10.00/10g

623577-60-6
43 suppliers
$45.00/10mg
75-30-9
278 suppliers
$15.00/1g

2302-25-2
255 suppliers
$6.00/1g

623577-60-6
43 suppliers
$45.00/10mg