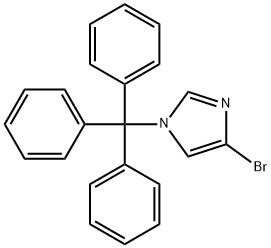
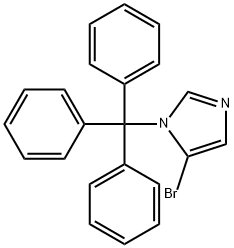
4-BROMO-1-TRITYL-1H-IMIDAZOLE synthesis
- Product Name:4-BROMO-1-TRITYL-1H-IMIDAZOLE
- CAS Number:87941-55-7
- Molecular formula:C22H17BrN2
- Molecular Weight:389.29
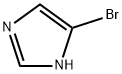
2302-25-2
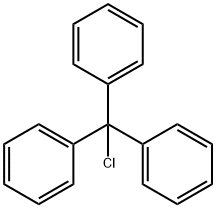
76-83-5

87941-55-7
To a 250 mL single neck flask was added 4-bromo-1H-imidazole (30 g, 205 mmol) and a solvent mixture of dichloromethane:tetrahydrofuran (1:1, v/v) with stirring at room temperature. Subsequently, triphenylchloromethane (62 g, 226 mmol) and triethylamine (29 mL) were slowly added. The reaction mixture was continued to be stirred at room temperature for 1 hour. Upon completion of the reaction, it was quenched by the addition of water and 1 N hydrochloric acid. The reaction mixture was extracted with dichloromethane, the organic layers were combined and dried with anhydrous sodium sulfate. Finally, the dichloromethane was removed by rotary evaporator to afford the target compound 4-bromo-1-trityl-1H-imidazole (61 g, 72% yield).
Yield:87941-55-7 72%
Reaction Conditions:
with triethylamine in tetrahydrofuran;dichloromethane at 20; for 1 h;
Steps:
1.4 (4) Synthesis of 4-bromo-1-trityl-1H-imidazole (Intermediate 7)
To a single-necked flask was added 4-bromoimidazole (30 g, 205 mmol)Dichloromethane: tetrahydrofuran = 1: 1,And triphenylchloromethane (62 g, 226 mmol)Triethylamine (29 ml) was added with stirring at room temperature,Continue stirring for 1 h, add water and 1N hydrochloric acid,Adding methylene chloride extraction,The organic layers were combined and dried over anhydrous sodium sulfate,Spin dry dichloromethane to give compound 7 (61g, 72% yield).
References:
Hinova Pharmaceuticals Inc.;Fan, Lei;Chen, Ke;Li, Xinghai;Chen, Yuanwei CN106256830, 2016, A Location in patent:Paragraph 0044; 0045; 0055; 0056; 0057
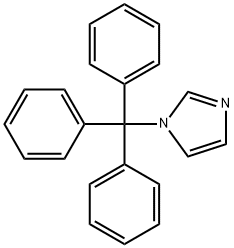
15469-97-3
209 suppliers
$9.00/250mg
121816-82-8
6 suppliers
inquiry

87941-55-7
87 suppliers
$69.00/1g

2302-25-2
255 suppliers
$6.00/1g

76-83-5
714 suppliers
$6.00/25g
76-84-6
457 suppliers
$6.00/25g

87941-55-7
87 suppliers
$69.00/1g