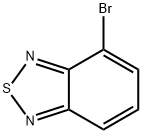
4-bromo-2,1,3-benzothiadiazole synthesis
- Product Name:4-bromo-2,1,3-benzothiadiazole
- CAS Number:22034-13-5
- Molecular formula:C6H3BrN2S
- Molecular Weight:215.07
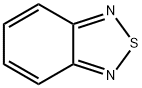
273-13-2

22034-13-5
General procedure for the synthesis of 4-bromo-2,1,3-benzothiadiazole from 2,1,3-benzothiadiazole: In a round-bottomed flask equipped with a reflux condenser, 25.0 g (183.7 mmol) of 2,1,3-benzothiadiazole and 150 mL of 48% hydrobromic acid solution were added. After heating the mixture to 100 °C, 8.5 mL (165.4 mmol) of bromine was added slowly and the reaction was kept at 100 °C with stirring for 9 hours, followed by cooling to room temperature. Upon completion of the reaction, 200 mL of dichloromethane was added to the reaction mixture to dissolve the precipitated solid, followed by 100 mL of aqueous sodium sulfate. The organic layer was separated, washed with saturated aqueous sodium bicarbonate, dried over anhydrous sodium sulfate, and subsequently concentrated under reduced pressure to remove the solvent to afford the crude product 4-bromo-2,1,3-benzothiadiazole. The crude product was suspended in 200 mL of hexane/ethyl acetate (4:1, v/v) mixed solvent and filtered to remove the insoluble by-product 4,7-dibromo-2,1,3-benzothiadiazole. The filtrate was concentrated and suspended again in 200 mL of hexane and filtered to collect the solid to give 11.2 g of 4-bromo-2,1,3-benzothiadiazole. The filtrate was further concentrated and purified by column chromatography using hexane/ethyl acetate (97:3, v/v) as eluent to afford 8.0 g of 4-bromo-2,1,3-benzothiadiazole. A total of 19.2 g (89.3 mmol) of product was obtained in 49% yield.
95-54-5
541 suppliers
$9.00/1g

22034-13-5
138 suppliers
$15.00/100mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1.1: thionyl chloride / toluene / 3 h / Reflux
1.2: 19 h / Reflux
2.1: hydrogen bromide; bromine / 3 h / Reflux
References:
Wa̧sik, Romualda;Wińska, Patrycja;Poznański, Jarosław;Shugar, David [Journal of Physical Chemistry B,2012,vol. 116,# 24,p. 7259 - 7268]
767-64-6
155 suppliers
$21.00/250mg

22034-13-5
138 suppliers
$15.00/100mg