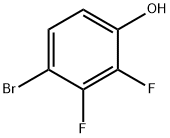
4-Bromo-2,3-difluorophenol synthesis
- Product Name:4-Bromo-2,3-difluorophenol
- CAS Number:144292-32-0
- Molecular formula:C6H3BrF2O
- Molecular Weight:208.99
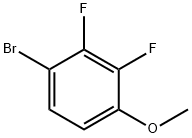
406482-22-2

144292-32-0
The general procedure for the synthesis of 2,3-difluoro-4-bromophenol from 4-bromo-2,3-difluoroanisole was as follows: to a stirred solution of 1-bromo-2,3-difluoro-4-methoxybenzene (3 g, 13.45 mmol) in dichloromethane (DCM, 30 mL) was slowly added dropwise to a dichloromethane (DCM) solution of 1 M boron tribromide (BBr3) (26.9) at -20 °C. The dropwise addition time was controlled to 10 min. mL, 26.9 mmol), and the dropwise addition time was controlled over 10 min. The cooling bath was removed and the reaction mixture was continued to be stirred at room temperature for 12 hours. Subsequently, the reaction mixture was cooled to 10 °C and the reaction was quenched with saturated aqueous sodium bicarbonate solution (100 mL). The mixture was diluted with dichloromethane (DCM, 150 mL) and the organic layer was separated, dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford the target product 4-bromo-2,3-difluorophenol (2 g, 9.47 mmol, 70% yield) in the form of a dark red oil. NMR hydrogen spectrum (400MHz, chloroform-d): δ 7.15-7.21 (m, 1H), 6.69-6.76 (m, 1H), 5.30-5.31 (m, 1H) ppm.
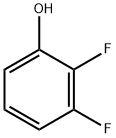
6418-38-8
339 suppliers
$10.00/1g

144292-32-0
192 suppliers
$7.00/1g
Yield: 73%
Reaction Conditions:
with acetic acid;potassium bromide in water;acetic acid at 35;regioselective reaction;
Steps:
4.2. General Procedure for the Bromination
General procedure: 0.11 g (1.0 mmol) of 4-methylphenol, 5 mL of acetic acid, 0.5 mL of water, and 0.12 g (1.0 mmol)potassium bromide were placed in round-bottomed flask. Then, 0.19 g (0.2 mmol)ZnAl-BrO3--LDHs was added in the flask under stirring at 35 °C. After the addition, stirring wascontinued to the end of reaction (monitored by thin layer chromatography). The residualZnAl-BrO3--LDHs were removed by centrifugation. The product was extracted with 3 × 10 mLdichloromethane. The combined extract was washed with sodium sulfite solution, brine, and dried(Na2SO4). Evaporation of the solvent left the crude product. The crude product was purified bycolumn chromatography over silica gel (ethyl acetate-petroleum ether) to obtain pure product.
References:
Wang, Ligeng;Feng, Chun;Zhang, Yan;Hu, Jun [Molecules,2020,vol. 25,# 4,art. no. 914] Location in patent:supporting information

406482-22-2
110 suppliers
$5.00/250mg

144292-32-0
192 suppliers
$7.00/1g