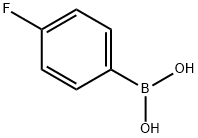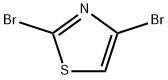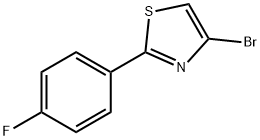
4-Bromo-2-(4-fluorophenyl)thiazole synthesis
- Product Name:4-Bromo-2-(4-fluorophenyl)thiazole
- CAS Number:1142196-38-0
- Molecular formula:C9H5BrFNS
- Molecular Weight:258.11
Yield:1142196-38-0 450 mg
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in 1,2-dimethoxyethane at 105; for 0.2 h;Inert atmosphere;Sealed tube;Microwave irradiation;Suzuki Coupling;
Steps:
16.1 Step 1.
Preparation of 4-bromo-2-(4-fluorophenyl)thiazole
Following a modified procedure (Bach, T.; Heuser, S. Tetrahedron Lett. 2000, 41, 1707), a mixture of 2,4-dibromothiazole (486 mg, 2.0 mmol), 4-fluorophenyl boronic acid (266 mg, 1.9 mmol), and aqueous 2.0 M Na2CO3 solution (2.3 mL, 4.6 mmol) in DME (6.8 mL) was sparged with Ar for 3 min. Tetrakis(triphenylphosphine)palladium(0) (150 mg) was added and the reaction mixture was sparged with Ar for 1 min.
The reaction was sealed and irradiated at 105° C. for 12 min in a microwave reactor.
The reaction mixture was partitioned with EtOAc (50 mL) and saturated aqueous NaHCO3 solution (10 mL).
The layers were separated and the organic layer was washed with saturated aqueous NaHCO3 solution (10 mL), brine (20 mL), dried (Na2SO4), and concentrated in vacuo.
The crude material was purified by column chromatography over silica gel to provide 450 mg of 4-bromo-2-(4-fluorophenyl)thiazole which was directly used in the next step without further purification: LCMS (m/z): 259.9 (MH+), tR=1.08 min.
References:
US2013/210818,2013,A1 Location in patent:Paragraph 0375