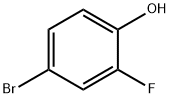
4-Bromo-2-fluorophenol synthesis
- Product Name:4-Bromo-2-fluorophenol
- CAS Number:2105-94-4
- Molecular formula:C6H4BrFO
- Molecular Weight:191
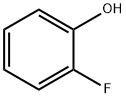
367-12-4

2105-94-4
Example 5: Preparation of methyl 2-[4-(4-bromo-2-fluorophenoxy)phenoxy]propionate (STR29) 1. 2-Fluorophenol (22.4 g, 0.2 mol) was dissolved in dichloromethane (250 mL) under ice bath conditions and cooled to about 3 °C. 2. 2. bromine (31.97 g, 0.2 mol) was added slowly, keeping the reaction temperature at ice bath conditions. 3. the reaction mixture was stirred at ice bath temperature for 2 hours, followed by continued stirring at room temperature for 1 hour. 4. the reaction mixture was quenched by pouring into water (600 mL) containing excess sodium bisulfite. 5. The organic phase was separated and the aqueous phase was washed with additional dichloromethane (200 mL). 6. The organic extracts were combined, washed with saturated sodium bicarbonate solution and subsequently dried over anhydrous magnesium sulfate. 7. The solvent was removed by evaporation to afford the target product 2-fluoro-4-bromophenol as a colorless oil (34.5 g, 90% yield). 8. The structure of the product was confirmed to be as expected by NMR hydrogen spectroscopy (CDCl3).

367-12-4
425 suppliers
$5.00/10g

2105-94-4
366 suppliers
$6.00/5g
Yield:2105-94-4 90%
Reaction Conditions:
with bromine in dichloromethane;water
Steps:
5 Preparation of 2-[4-(4-bromo-2-fluorophenoxy)phenoxy]propionic acid methyl ester STR29
EXAMPLE 5 Preparation of 2-[4-(4-bromo-2-fluorophenoxy)phenoxy]propionic acid methyl ester STR29 To a stirred solution of 2-fluorophenol (22.4 g, 0.2 mol) in methylene chloride (250 ml) which was cooled to ~3° C. in an ice bath, was added, all at once, bromine (31.97 g, 0.2 mol). The resulting solution was stirred at ice bath temperature for two hours and then at room temperature for 1 hour. The mixture was poured into water (600 ml) containing excess sodium bisulfite. The organic phase was separated and the aqueous phase was washed with additional methylene chloride (200 ml). The combined organic extracts were washed with saturated sodium bicarbonate, dried (MgSO4) and the solvent evaporated to give the desired 2-fluoro-4-bromophenol as a colorless oil (34.5 g, 90%). The NMR (CDCl3) was consistent with the assigned structure.
References:
The Dow Chemical Company US4642338, 1987, A
106-41-2
498 suppliers
$13.00/5g

2105-94-4
366 suppliers
$6.00/5g
2357-52-0
318 suppliers
$6.00/5g

2105-94-4
366 suppliers
$6.00/5g

367-12-4
425 suppliers
$5.00/10g

2105-94-4
366 suppliers
$6.00/5g
576-86-3
123 suppliers
$21.00/5g
2040-89-3
181 suppliers
$6.00/1g