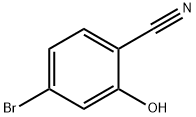
4-BROMO-2-HYDROXYBENZONITRILE synthesis
- Product Name:4-BROMO-2-HYDROXYBENZONITRILE
- CAS Number:288067-35-6
- Molecular formula:C7H4BrNO
- Molecular Weight:198.02
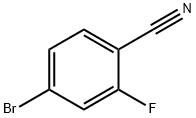
105942-08-3

288067-35-6
General procedure for the synthesis of 4-bromo-2-hydroxybenzonitrile from 4-bromo-2-fluorobenzonitrile: 2-fluoro-5-bromobenzonitrile (30 g, 152.3 mmol), potassium acetate (222.4 g, 228.5 mmol), and 18-crown-6 ether (60.4 g, 228.5 mmol) were dissolved in acetonitrile (400 mL) and heated and refluxed for 36 hours. After completion of the reaction, the mixture was cooled to room temperature, 2.5 N NaOH solution (200 mL) was added and stirred overnight at room temperature. The reaction mixture was extracted with ether and the organic layer was discarded. The aqueous layer was acidified with 6 N HCl and extracted with ethyl acetate. The organic layers were combined, dried with anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product was purified by fast column chromatography (40% ethyl acetate/hexane) to afford 4-bromo-2-hydroxybenzonitrile as a light yellow foamy solid (24.45 g, 81% yield). NMR hydrogen spectrum (300 MHz, DMSO-d6): δ 7.13 (dd, J = 1.7, 8.3 Hz, 1H), 7.17 (d, J = 1.7 Hz, 1H), 7.61 (d, J = 8.3 Hz, 1H).
179897-89-3
317 suppliers
$10.00/5g

288067-35-6
164 suppliers
$5.00/250mg
Yield:288067-35-6 81%
Reaction Conditions:
with sodium hydroxide;potassium acetate;18-crown-6 ether in ethyl acetate;acetonitrile
Steps:
1.a a
a 2-Hydroxyl-4-bromobenzonitrile A mixture of 2-fluoro-5-bromobenzonitrile (30 g, 152.3 mmole), potassium acetate (222.4 g, 228.5 mmole), and 18-crown-6 ether (60.4 g, 288.5 mmole) in MeCN (400 mL) was heated at reflux in 36 h. The mixture was cooled, added 2.5 N NaOH (200 mL), stirred at RT overnight. The mixture was extracted with ether (discarded). The aqueous layer was acidified with 6N HCl, extracted with EtOAc, dried over MgSO4, concentrated, purified by flash column chromatography (40% EtOAc/Hex) to give a light yellow foam (24.45 g, 81%). 1H-NMR (300 MHz, DMSO-d6): δ7.13 (dd, J=1.7, 8.3 Hz, 1H), 7.17 (d, J=1.7 Hz, 1H), 7.61 (d, J=8.3 Hz, 1H).
References:
SmithKline Beecham Corporation US6417215, 2002, B1

105942-08-3
382 suppliers
$7.00/5g

288067-35-6
164 suppliers
$5.00/250mg
330793-38-9
149 suppliers
inquiry

288067-35-6
164 suppliers
$5.00/250mg
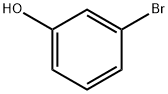
591-20-8
349 suppliers
$10.00/1g

288067-35-6
164 suppliers
$5.00/250mg