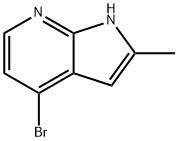
4-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine synthesis
- Product Name:4-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine
- CAS Number:1014613-64-9
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06
1014613-05-8
![4-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/1014613-64-9.gif)
1014613-64-9
General procedure for the synthesis of 4-bromo-2-methyl-1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine from 4-bromo-2-methyl-1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine: To 4-bromo-2-methyl-1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine (16 g, 0.045 mol) of 1,4-dioxane ( 320 mL) solution was added to 2M aqueous sodium hydroxide solution (114 mL, 0.228 mol). The reaction mixture was heated to 60°C and kept for 16 hours. After the reaction was completed, the mixture was cooled to room temperature. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give 4-bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine as a milky white solid (10.07 g, 104% yield). lC/MS (Method B): m/z 211/213 [M+H]+, retention time 1.04 min.

1014613-05-8
48 suppliers
$200.00/50mg
![4-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/1014613-64-9.gif)
1014613-64-9
72 suppliers
$45.00/100mg
Yield: 100%
Reaction Conditions:
with hydrogenchloride;water in 1,4-dioxane at 60; for 16 h;Inert atmosphere;
Steps:
10
Description 10 4-bromo-2-methyl-1W-pyrrolo[2,3-b]pyridine To a solution of 4-bromo-2-methyl-1 -(phenylsulfonyl)-1 H-pyrrolo[2,3-5]pyridine (D9) (16 g, 0.045 mol) in 1 ,4 dioxane (320 mL) was added 2M aqueous sodium hydroxide (114 rmL, 0.228 mol).The reaction was heated to 6O0C for 16 h. The reaction was cooled to ambient temperature. The organic layer was separated and the aqueous extracted with ethylacetate. The combined organics were washed with brine and dried over magnesium sulphate, filtered and concentrated in vacuo to give the title compound as a cream solid (10.07 g, 104%) LC/MS (Method B): MH+ 21 1/213 seen at retention time 1.04 mins.
References:
GLAXO GROUP LIMITED WO2009/112475, 2009, A1 Location in patent:Page/Page column 62
![1H-Pyrrolo[2,3-b]pyridine, 4-bromo-2-methyl-1-[(4-methylphenyl)sulfonyl]-](/CAS/20200515/GIF/1415928-58-3.gif)
1415928-58-3
0 suppliers
inquiry
![4-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/1014613-64-9.gif)
1014613-64-9
72 suppliers
$45.00/100mg
![4-BROMO-1-(PHENYLSULFONYL)-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/889939-25-7.gif)
889939-25-7
96 suppliers
$38.00/250mg
![4-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/1014613-64-9.gif)
1014613-64-9
72 suppliers
$45.00/100mg
348640-06-2
337 suppliers
$6.00/250mg
![4-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/1014613-64-9.gif)
1014613-64-9
72 suppliers
$45.00/100mg
![4-BROMO-1-[(4-METHYLPHENYL)SULFONYL]-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/348640-07-3.gif)
348640-07-3
112 suppliers
$47.00/1g
![4-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/1014613-64-9.gif)
1014613-64-9
72 suppliers
$45.00/100mg