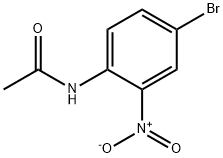
4'-BROMO-2'-NITROACETANILIDE synthesis
- Product Name:4'-BROMO-2'-NITROACETANILIDE
- CAS Number:881-50-5
- Molecular formula:C8H7BrN2O3
- Molecular Weight:259.06
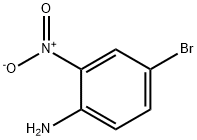
875-51-4

108-24-7

881-50-5
The general procedure for the synthesis of 4-bromo-2-nitro-N-acetylaniline from 4-bromo-2-nitroaniline and acetic anhydride was as follows: 4-bromo-2-nitroaniline (30.3 g, 138 mmol) was dissolved in 240 mL of acetic acid, followed by the addition of acetic anhydride (22.44 g, 220.2 mmol). The reaction mixture was heated to 95 °C and maintained at this temperature for 7.5 hours. Upon completion of the reaction, the reaction solution was cooled to room temperature and slowly poured into 600 mL of ice water. After the ice was completely melted, it was extracted with dichloromethane (90 mL x 3). The resulting solid was dissolved in 600 mL of dichloromethane, and all organic phases were combined and dried with anhydrous sodium sulfate. Finally, the target product N-(4-bromo-2-nitrophenyl)acetamide (35.4 g, orange solid) was obtained in 99.1% yield by concentrating the organic phase.
Yield:881-50-5 99.1%
Reaction Conditions:
with acetic acid at 95; for 7.5 h;
Steps:
3.1 N-(4-bromo-2-nitrophenyl)acetamide
4-Bromo-2-nitroaniline 3a (30.3 g, 138 mmol) was dissolved in 240 mL of acetic acid, and acetic anhydride (22.44g, 220.2 mmol) was added, and the mixture was heated to 95 ° C for 7.5 hours. The reaction solution was cooled to room temperature and poured into 600 mL of ice water. After ice-melting, it was extracted with dichloromethane (90 mL×3), and the solid was dissolved in dichloromethane (600 mL), the organic phase was combined, dried over anhydrous sodium sulfate Concentration gave N-(4-bromo-2-nitrophenyl)acetamide 3b (35.4g, orange solid), yield: 99.1%.
References:
CN108264511,2018,A Location in patent:Paragraph 0243; 0246-0249
103-88-8
273 suppliers
$6.00/5g

881-50-5
67 suppliers
$8.00/100mg

108-24-7
0 suppliers
$14.00/250ML

106-40-1
483 suppliers
$12.00/5g

881-50-5
67 suppliers
$8.00/100mg
552-32-9
112 suppliers
$20.00/1g

881-50-5
67 suppliers
$8.00/100mg
