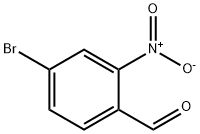
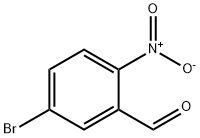
4-Bromo-2-nitrobenzaldehyde synthesis
- Product Name:4-Bromo-2-nitrobenzaldehyde
- CAS Number:5551-12-2
- Molecular formula:C7H4BrNO3
- Molecular Weight:230.02
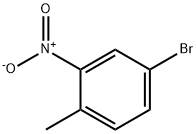
60956-26-5

5551-12-2
Step A: Concentrated sulfuric acid (324 mL) was added slowly and dropwise to a solution of 4-bromo-1-methyl-2-nitrobenzene (300 g, 1.38 mol) in acetic anhydride (2400 mL) at 0 °C. Subsequently, a solution of chromium trioxide (384 g, 3.84 mol) dissolved in acetic anhydride (2160 mL) was added, keeping the internal temperature of the reaction system below 10 °C during the process. The reaction mixture was stirred for 1 hour and then slowly poured into an ice-water mixture. The solid product was collected by filtration and washed with plenty of water until the washings were colorless. The resulting solid was suspended in 1800 mL of a 2% aqueous sodium carbonate solution, stirred thoroughly and then filtered again, washed with water and finally dried under reduced pressure. [000195] The diacetate product obtained above was suspended in a mixed solution comprising 1360 mL of concentrated hydrochloric acid, 1250 mL of water and 480 mL of ethanol and refluxed for 45 minutes. After the reaction was completed, it was cooled to room temperature, the solid product was collected by filtration and washed with water. The final 4-bromo-2-nitrobenzaldehyde (147 g, 45% overall yield in two steps) was obtained as a brown solid, which could be used in the subsequent reaction without further purification.
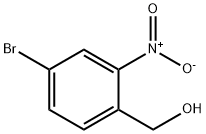
22996-19-6
127 suppliers
inquiry

5551-12-2
170 suppliers
$10.00/1g
Yield:5551-12-2 78%
Reaction Conditions:
with manganese(IV) oxide in dichloromethane at 20; for 12 h;
Steps:
25.2
Step 2. 4-Bromo-2-nitrobenzaldehydeManganese (IV) oxide (4eq) was added to a 0.18M solution of (4-bromo-2-nitrophenyl)methanol in dichloromethane. The suspension was stirred at ambient temperature under Argon for 12 h. The reaction mixture was filtered through celite and filter cake was washed with dichloromethane. The combined filtrate was concentrated to give brown color solid in 78% yield.
References:
WO2007/117607,2007,A2 Location in patent:Page/Page column 333

60956-26-5
301 suppliers
$8.00/10g

5551-12-2
170 suppliers
$10.00/1g

135308-80-4
0 suppliers
inquiry

5551-12-2
170 suppliers
$10.00/1g

78508-22-2
6 suppliers
inquiry

5551-12-2
170 suppliers
$10.00/1g
552-89-6
655 suppliers
$5.00/1g

5551-12-2
170 suppliers
$10.00/1g

56990-05-7
0 suppliers
inquiry
20357-20-4
154 suppliers
$5.00/250mg
20357-21-5
156 suppliers
$31.00/100mg