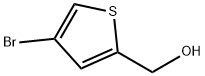
(4-BROMO-2-THIENYL)METHANOL synthesis
- Product Name:(4-BROMO-2-THIENYL)METHANOL
- CAS Number:79757-77-0
- Molecular formula:C5H5BrOS
- Molecular Weight:193.06
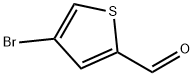
18791-75-8

79757-77-0
Step I: Sodium borohydride (5.20 g, 0.137 mol) was added batchwise to a solution of 4-bromo-2-thiophenecarboxaldehyde (25.0 g, 0.131 mol) in anhydrous tetrahydrofuran (400 mL). The reaction mixture was stirred at room temperature for 1.5 hours. Upon completion of the reaction, saturated ammonium chloride solution (100 mL) was slowly added to quench the reaction. Subsequently, the reaction mixture was extracted with ethyl acetate and the organic phases were combined and washed with saturated brine. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford (4-bromo-2-thienyl)methanol (25.02 g, 99% yield), and the product could be used for subsequent reactions without further purification. NMR hydrogen spectrum (400 MHz, CDCl3): δ = 1.93 (broad single peak, 1H), 4.79 (single peak, 2H), 6.93 (single peak, 1H), 7.18 (double peak, J = 1.5 Hz, 1H).

18791-75-8
287 suppliers
$9.00/5g
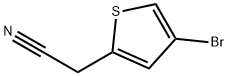
160005-43-6
29 suppliers
inquiry

79757-77-0
109 suppliers
$6.00/1g
Yield:79757-77-0 96%
Reaction Conditions:
with sodium borohydrid in ethanol;water
Steps:
R.5 [Preparation of 4-Bromothiophene-2-Ylacetonitrile]
Referential Example 5 [Preparation of 4-Bromothiophene-2-Ylacetonitrile] To a solution of 4-bromothiophene-2-carboxaldehyde (9.55 g, 50 mmol, purchased from Aldrich) in 100 ml of ethanol was added sodium borohydride (3.78 g, 100 mmol purchased from Yoneyama Yakuhin) gradually under cooling in an ice bath. After the completion of addition, the mixture was stirred further for 1.5 hours at room temperature. The reaction mixture was acidified with hydrochlorid acid, then was concentrated to dryness under reduced pressure. To the residue was added water and extracted with ether to give 9.29 g (yield: 96%) of 4-bromothiophen-2-ylmethanol as an oil.
References:
Morinaga Milk Industry Co., Ltd. US5589506, 1996, A

18791-75-8
287 suppliers
$9.00/5g

79757-77-0
109 suppliers
$6.00/1g