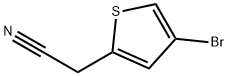
(4-Bromo-thiophen-2-yl)-acetonitrile synthesis
- Product Name:(4-Bromo-thiophen-2-yl)-acetonitrile
- CAS Number:160005-43-6
- Molecular formula:C6H4BrNS
- Molecular Weight:202.07
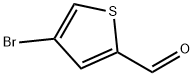
18791-75-8
287 suppliers
$9.00/5g

160005-43-6
29 suppliers
inquiry
79757-77-0
111 suppliers
$6.00/1g
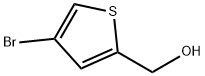
Yield: 96%
Reaction Conditions:
with sodium borohydrid in ethanol;water
Steps:
R.5 [Preparation of 4-Bromothiophene-2-Ylacetonitrile]
Referential Example 5 [Preparation of 4-Bromothiophene-2-Ylacetonitrile] To a solution of 4-bromothiophene-2-carboxaldehyde (9.55 g, 50 mmol, purchased from Aldrich) in 100 ml of ethanol was added sodium borohydride (3.78 g, 100 mmol purchased from Yoneyama Yakuhin) gradually under cooling in an ice bath. After the completion of addition, the mixture was stirred further for 1.5 hours at room temperature. The reaction mixture was acidified with hydrochlorid acid, then was concentrated to dryness under reduced pressure. To the residue was added water and extracted with ether to give 9.29 g (yield: 96%) of 4-bromothiophen-2-ylmethanol as an oil.
References:
Morinaga Milk Industry Co., Ltd. US5589506, 1996, A

18791-75-8
287 suppliers
$9.00/5g

79757-77-0
111 suppliers
$6.00/1g

160005-43-6
29 suppliers
inquiry
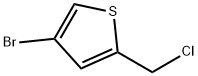
170859-70-8
44 suppliers
$45.00/100mg
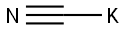
151-50-8
0 suppliers
$36.39/100g

160005-43-6
29 suppliers
inquiry

18791-75-8
287 suppliers
$9.00/5g

160005-43-6
29 suppliers
inquiry

79757-77-0
111 suppliers
$6.00/1g

160005-43-6
29 suppliers
inquiry