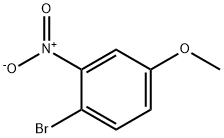
4-Bromo-3-nitroanisole synthesis
- Product Name:4-Bromo-3-nitroanisole
- CAS Number:5344-78-5
- Molecular formula:C7H6BrNO3
- Molecular Weight:232.03
96-96-8

5344-78-5
The general procedure for the synthesis of 4-bromo-3-nitroanisole from ice-dyed diazo component 1 was as follows: a solution of sodium nitrite (11.8 g) in water (28 mL) was slowly added dropwise to a 40% solution of nitroaniline (125 mmol) in hydrobromic acid (110 g) over a period of 0.5 h. The temperature of the reaction was maintained at 10 °C. The reaction mixture was stirred at 0-10 °C for 40 min and then filtered. Subsequently, the filtrate was added dropwise to a purple solution of copper(I) bromide (209 mmol) in hydrobromic acid (74 mL) at 0 °C over a period of 1 hour. The reaction mixture was gradually warmed to room temperature and held for 30 min, then warmed to 60 °C for 0.5 h. The reaction was finally heated and refluxed for 1 h. The reaction was carried out at a temperature of 0.5 °F to 0.5 °F. After completion of the reaction, the mixture was partitioned between water (2.0 L) and dichloromethane (600 mL) and the aqueous phase was then extracted with dichloromethane (300 mL). All organic layers were combined and washed sequentially with 10% sodium hydroxide solution (200 mL), water (600 mL), 10% hydrochloric acid (300 mL) and water (600 mL). The organic layer was dried over magnesium sulfate and concentrated to give 4-bromo-3-nitroanisole in 83% yield as a yellow oil.
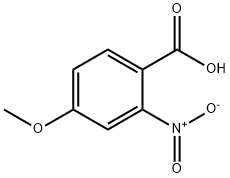
33844-21-2
229 suppliers
$15.00/1 g

5344-78-5
246 suppliers
$6.00/5g
Yield:5344-78-5 67%
Reaction Conditions:
with 2.9-dimethyl-1,10-phenanthroline;oxygen;copper (I) acetate;silver sulfate;sodium bromide in dimethyl sulfoxide at 160; for 24 h;Schlenk technique;
Steps:
26 Example 26. Synthesis of 2-nitro-4-methoxy bromobenzene.
Silak reaction tube equipped with a magnetic stirrer was charged with 6.2 mg of silver sulfate,36.3 mg of copper acetate, 12.5 mg of 2,9-dimethyl-1,10-o-phenanthroline,39.4 mg of 2-nitro-4-methoxybenzoic acid and 30.9 mg of sodium bromide4 mL of dimethyl sulfoxide. Heat 160 ° C in the presence of oxygenReaction for 24 hours. After the reaction was completed, distilled water was added to quench the reaction,Extraction with ethyl acetate 3 times, each time 10mL,The combined organic phases are concentrated,31.1 mg of 2-nitro-4-methoxybromobenzene was obtained in a yield of 67%.
References:
Nanchang University;Fu Zhengjiang;Cai Hu;Li Zhaojie;Jiang Ligao CN107325002, 2017, A Location in patent:Paragraph 0118

96-96-8
0 suppliers
$7.00/1g

5344-78-5
246 suppliers
$6.00/5g
577-72-0
150 suppliers
$45.00/50mg

5344-78-5
246 suppliers
$6.00/5g

33844-21-2
229 suppliers
$15.00/1 g

5344-78-5
246 suppliers
$6.00/5g
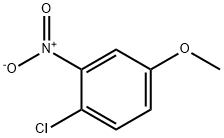
10298-80-3
337 suppliers
$10.00/10g
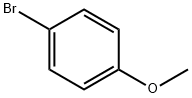
104-92-7
433 suppliers
$10.00/10g
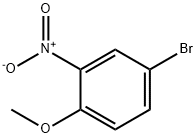
33696-00-3
206 suppliers
$10.00/1g

5344-78-5
246 suppliers
$6.00/5g