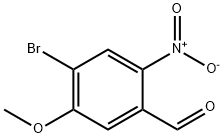
4-bromo-5-methoxy-2-nitrobenzaldehyde synthesis
- Product Name:4-bromo-5-methoxy-2-nitrobenzaldehyde
- CAS Number:1196664-96-6
- Molecular formula:C8H6BrNO4
- Molecular Weight:260.04
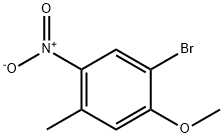
861076-28-0
69 suppliers
$79.00/250mg

1196664-96-6
9 suppliers
inquiry
Yield:1196664-96-6 54%
Reaction Conditions:
Stage #1: 1-bromo-2-methoxy-4-methyl-5-nitrobenzenewith N,N-dimethyl-formamide dimethyl acetal in N,N-dimethyl-formamide at 140; for 16 h;
Stage #2: with sodium periodate in water;N,N-dimethyl-formamide at 0; for 8 h;
Steps:
To a stirred solution of compound 12 (2.3 g, 9.4 mmol) in dry DMF (15 mL) was added N,N-dimethylformamide dimethyl acetal (DMF DMA) (6.0 g, 24.5 mmol). After the reaction mixture was heated at 140 °C for 16 h, the dark red solution was cooled to 0 °C and added rapidly to a stirred solution of NaI04 (10.5 g, 49 mmol) in H20/DMF (4: 1 , 25 mL) at 0 °C. After 8 h of stirring, the brown solution was filtered, and the reaction flask was rinsed with toluene/EtOAc (1 :1, 30 mL) and again filtered. The filtrate was washed with saturated aqueous NaCl solution (45 mL) and extracted with EtOAc (2 x 45 mL). The organic phase was dried (Na2S04) and concentrated, and the residue was purified by flash chromatography (hexane/EtOAc 3: 1) to afford aldehyde 13 (1.3 g, 54%): /0.22 (hexane/EtOAc 3: 1); 1H NMR (CDC13) δ 4.09 (s, 3H), 7.38 (s, 1H), 8.43 (s, 1H), 10.50 (s, 1H); 13C NMR (CDC13) δ 57.4, 1 10.5, 1 16.8, 130.3, 132.6, 142.3, 160.5, 187.7; ESI-HRMS [M-H]" C8H579BrN04 calcd for m/z 257.9402, found 257.9407.
References:
WO2011/56599,2011,A2 Location in patent:Page/Page column 33-34
5367-32-8
187 suppliers
$10.00/5g

1196664-96-6
9 suppliers
inquiry
452-74-4
315 suppliers
$17.00/25g

1196664-96-6
9 suppliers
inquiry
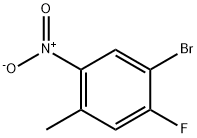
224185-19-7
204 suppliers
$7.00/1g

1196664-96-6
9 suppliers
inquiry