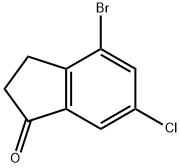
4-Bromo-6-chloro-indan-1-one synthesis
- Product Name:4-Bromo-6-chloro-indan-1-one
- CAS Number:1260017-94-4
- Molecular formula:C9H6BrClO
- Molecular Weight:245.5
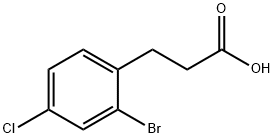
66192-04-9

1260017-94-4
The general procedure for the synthesis of 4-bromo-6-chloro-2,3-dihydro-1H-inden-1-one using 3-(2-bromo-4-chlorophenyl)propionic acid as starting material was as follows: oxalyl chloride (3.6 mL) was added slowly and dropwise under stirring to a solution of 3-(2-bromo-4-chlorophenyl)propionic acid (4.4 g) in dichloromethane (40 mL). The reaction mixture was stirred continuously at 40 °C for 2 hours. Subsequently, aluminum trichloride (AlCl3, 2.7 g) was added to the reaction system and the mixture was heated to reflux and maintained for 1 hour. Upon completion of the reaction, the mixture was cooled to room temperature and partitioned between ethyl acetate and saturated aqueous sodium bicarbonate (NaHCO3). The aqueous phase was extracted twice with ethyl acetate, all organic phases were combined and washed with brine. The organic layer was dried over anhydrous sodium sulfate (Na2SO4) and evaporated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography with the eluent cyclohexane/ethyl acetate (95:5→90:10) to afford the target compound 4-bromo-6-chloro-2,3-dihydro-1H-inden-1-one. Yield: 3.7 g; liquid chromatography (Method 1): retention time (tR) = 1.20 min; mass spectrometry (ESI+): m/z = 245 [M + H]+.

66192-04-9
14 suppliers
inquiry

1260017-94-4
29 suppliers
$45.00/10mg
Yield:1260017-94-4 3.7 g
Reaction Conditions:
with aluminum (III) chloride;oxalyl dichloride in dichloromethane at 40; for 3 h;Reflux;
Steps:
4 Step 4: 4-Bromo-6-chloro-2,3-dihydro-1 H-inden-1 -one
Oxalylchloride (3.6 ml_) is added dropwise to a solution of 3-(2-bromo-4- chlorophenyl)propanoic acid (4.4 g) in dichloromethane (40 ml_). The m ixture is stirred for 2 hours at 40°C and then treated with AICI3 (2.7 g). The mixture is heated to reflux for 1 hour, cooled to room temperature and partitioned between ethyl acetate and saturated aqueous NaHCO3 solution. The aqueous phase is extracted twice with ethyl acetate and the combined organic phases are washed with brine. After drying (Na2SO4) the solvents are evaporated. The residue is chromatographed on silica gel (cyclohexane/ethyl acetate 95:5→90:10) to give the title compound. Yield: 3.7 g; LC (method 1 ): tR = 1 .20 min; Mass spectrum (ESI+): m/z = 245 [M+H]+.
References:
WO2013/144098,2013,A1 Location in patent:Page/Page column 123

66191-92-2
2 suppliers
inquiry

1260017-94-4
29 suppliers
$45.00/10mg
2019-34-3
228 suppliers
$6.00/1g

1260017-94-4
29 suppliers
$45.00/10mg