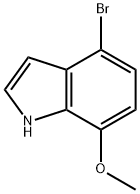
4-BROMO-7-METHOXY-1H-INDOLE synthesis
- Product Name:4-BROMO-7-METHOXY-1H-INDOLE
- CAS Number:436091-59-7
- Molecular formula:C9H8BrNO
- Molecular Weight:226.07
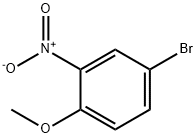
33696-00-3
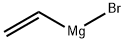
1826-67-1

436091-59-7
Example 13 Synthesis of 4-bromo-7-methoxyindole (LII): to a tetrahydrofuran (THF) solution (300 ml) of 4-bromo-2-nitroanisole (7.89 g, 33.3 mmol) was slowly added vinylmagnesium bromide (1 M in THF) at -60 °C. The reaction temperature was kept below -40 °C while the reaction mixture was stirred so that the temperature was gradually raised to -40 °C within 2 hours. After completion of the reaction, the reaction was quenched with saturated ammonium chloride (NH4Cl) solution (100 ml, 0.1 mol). The aqueous phase was extracted with ether (Et2O, 200 ml), the organic layers were combined, washed with brine, dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated. Finally, the target product 4-bromo-7-methoxyindole (LII, 594 mg, 8% yield) was purified by fast chromatography.
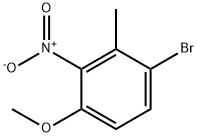
85598-13-6
34 suppliers
inquiry
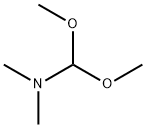
4637-24-5
647 suppliers
$5.00/25g

436091-59-7
88 suppliers
$45.00/50mg
Yield:436091-59-7 44%
Reaction Conditions:
Stage #1:1-bromo-4-methoxy-2-methyl-3-nitro-benzene;N,N-dimethyl-formamide dimethyl acetal with pyrrolidine in N,N-dimethyl-formamide at 90; for 18 h;
Stage #2: with iron;acetic acid for 1 h;Reflux;
Stage #3: with sodium carbonate in water
Steps:
B-5.3 Step 3 Preparation of 4-bromo-7-methoxyindole:
To a solution of 1-bromo-4-methoxy-2-methyl-3-nitro-benzene (31 g, 0.126 mol) in DMF (150 mL) was added dimethylformamide dimethylacetal (27 mL) and pyrrolidine (10.5mL, 0.127 mol). The mixture was heated at 90°C for 18h and cooled to r.t. The mixture was diluted with water and extracted with CH2CI2. The organic phase was dried over Na2S04i filtered and concentrated. The residue was dissolved into HOAc (50 mL) and added dropwise to a solution of Fe (20.5 g, 0.37 mol) in boiling HOAc (150 mL). The mixture was refluxed for 1h and cooled to r.t., water added and the mixture was neutralised with Na2C03, and extracted with CH2CI2.The organic phase was dried over Na2S04, filtered and concentrated. The residue was purified by Prep-TLC (PE/EtOAc =5/1) to give the title compound (13 g, 44%). H NMR CDCI3400 MHz δ: 8.41 (brs, 1 H), 7.18-7.08 (m, 2H), 6.49-6.43 (m, 2H), 3.86 (s, 3H).
References:
SAREUM LIMITED;READER, John Charles WO2013/117522, 2013, A1 Location in patent:Page/Page column 41-42

85598-13-6
34 suppliers
inquiry

436091-59-7
88 suppliers
$45.00/50mg

33696-00-3
206 suppliers
$10.00/1g
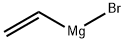
1826-67-1
209 suppliers
$51.29/100ml

436091-59-7
88 suppliers
$45.00/50mg
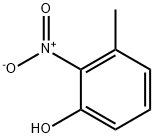
4920-77-8
225 suppliers
$15.00/5g

436091-59-7
88 suppliers
$45.00/50mg
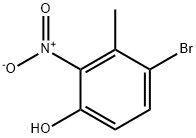
85598-12-5
48 suppliers
$21.00/100mg

436091-59-7
88 suppliers
$45.00/50mg