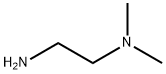
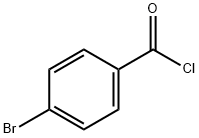
4-bromo-N-[2-(dimethylamino)ethyl]benzamide synthesis
- Product Name:4-bromo-N-[2-(dimethylamino)ethyl]benzamide
- CAS Number:301678-39-7
- Molecular formula:C11H15BrN2O
- Molecular Weight:271.15
Yield:301678-39-7 94%
Reaction Conditions:
Stage #1: 4-Bromobenzoic acidwith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in N,N-dimethyl-formamide at 20; for 0.333333 h;
Stage #2: N,N-dimethylethylenediamine in N,N-dimethyl-formamide at 20; for 1 h;
Steps:
7.i
Step (i) of Reference Example 7: 4-Bromo-N-(2-(dimethylamino)ethyl)benzamide 4-Bromophenylcarboxylic acid (100.0 mg, 0.497 mmol), 100.8 mg (0.746 mmol) of 1-hydroxybenzotriazole, 143.0 mg (0.746 mmol) of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, and N,N-dimethylformamide (1.0 ml) were added in that order, and the mixture was stirred at room temperature for 20 min. Thereafter, 0.080 ml (0.746 mmol) of N',N'-dimethylethane-1,2-diamine was added thereto, and the mixture was stirred at room temperature for one hr. A saturated aqueous sodium hydrogencarbonate solution was added thereto, and the mixture was then extracted with ethyl acetate. The extract was dried over anhydrous sodium sulfate and was then filtered. The filtrate was concentrated under the reduced pressure, and the residue was purified by preparative thin-layer chromatography (chloroform : methanol : 28% aqueous ammonia = 9 : 2 : 0.2) to give 127 mg (yield 94%) of the title compound. 1H-NMR (400 MHz, CDCl3) δ: 2.31 (6H, s), 2.57 (2H, t, J = 6.8 Hz), 3.52 (2H, t, J = 6.8 Hz), 7.62 (2H, ddd, J = 2.0, 2.2, 8.6 Hz), 7.75 (2H, ddd, J = 1.9, 2.2, 8.8 Hz
References:
EP2151447,2010,A1 Location in patent:Page/Page column 43
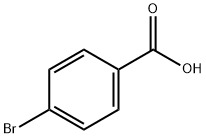
586-76-5
495 suppliers
$10.00/1g
![4-bromo-N-[2-(dimethylamino)ethyl]benzamide](/CAS/20180906/GIF/301678-39-7.gif)
301678-39-7
7 suppliers
inquiry