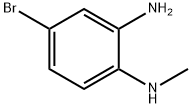
4-broMo-N1-Methylbenzene-1,2-diaMine synthesis
- Product Name:4-broMo-N1-Methylbenzene-1,2-diaMine
- CAS Number:69038-76-2
- Molecular formula:C7H9BrN2
- Molecular Weight:201.06
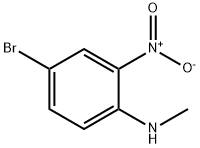
53484-26-7

69038-76-2
General procedure for the synthesis of 4-bromo-N1-methylbenzene-1,2-diamine from 4-bromo-N-methyl-2-nitroaniline: to a solution of 4-bromo-N-methyl-2-nitroaniline (2.1 g, 9 mmol) in methanol (MeOH, 50 mL) was added iron powder (Fe, 2.5 g, 45 mmol) and ammonium chloride (NH4Cl, 4.8 g, 90 mmol). The reaction mixture was stirred at 80 °C for 4 hours. After completion of the reaction, the mixture was poured into water (H2O, 60 mL) and extracted with ethyl acetate (EA, 40 mL × 2). The organic layers were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure to give 4-bromo-N1-methylbenzene-1,2-diamine (2.0 g, 99% yield) as a yellow solid.LC-MS m/z: 203.1 [M + H]+. Retention time (tR) = 1.64 min.

53484-26-7
85 suppliers
$30.00/250mg

69038-76-2
74 suppliers
$28.00/100mg
Yield: 99%
Reaction Conditions:
with iron;ammonium chloride in methanol at 80; for 4 h;
Steps:
To a solution of 4-bromo-N-methyl-2-nitroaniline (2.1 g, 9 mmol) in MeOH (50mL) was added Fe powder (2.5 g, 45 mmol) and NH4C1 (4.8 g, 90 mmol). The mixture wasstirred at 80 °C for 4 h, poured into H20 (60 mL) and extracted with EA (40 mL x 2). The organic layers were dried over anhydrous Na2SO4, filtered and concentrated in vacuo to afford 4-bromo-N’-methylbenzene-1,2-diamine (2.0 g, 99 %) as a yellow solid. LC-MS m/z: 203.1 [M+Hj . tR = 1.64 min
References:
LYSOSOMAL THERAPEUTICS INC.;SKERLJ, Renato, T.;BOURQUE, Elyse Marie Josee;LANSBURY, Peter, T.;GOOD, Andrew, C. WO2017/176960, 2017, A1 Location in patent:Paragraph 00743
364-73-8
278 suppliers
$8.00/10g

74-89-5
0 suppliers
$13.44/25ML

69038-76-2
74 suppliers
$28.00/100mg

364-73-8
278 suppliers
$8.00/10g

69038-76-2
74 suppliers
$28.00/100mg
875-51-4
306 suppliers
$5.00/1g

69038-76-2
74 suppliers
$28.00/100mg
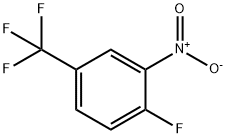
367-86-2
249 suppliers
$5.00/5g

69038-76-2
74 suppliers
$28.00/100mg