
4-BROMO-TOLUENE-3-SULFONYL CHLORIDE synthesis
- Product Name:4-BROMO-TOLUENE-3-SULFONYL CHLORIDE
- CAS Number:141113-98-6
- Molecular formula:C7H6BrClO2S
- Molecular Weight:269.54
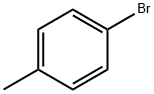
106-38-7
410 suppliers
$14.00/5g

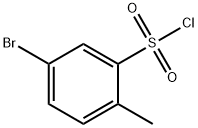
141113-98-6
20 suppliers
$76.00/250mg
69321-56-8
78 suppliers
$67.00/100mg
Yield:69321-56-8 81%
Reaction Conditions:
with chlorosulfonic acid in dichloromethane;
Steps:
4.11. 5-Bromo-2-methylbenzenesulfonyl chloride (23)
A solution of ClSO3H (7.00 mL, 103 mmol) in CH2Cl2 (12 mL) wasadded dropwise to an ice-cold solution of 4-bromotoluene (2.61 g,15 mmol) in CH2Cl2 (25 mL). The mixture was stirred in an ice-bathovernight, while the temperature increased to 10 °C. The solvent wasremoved and the residue was added dropwise to ice-water. The formedsolid was filtered off and washed with water to afford a mixture (3.85 g,94%, 86:14 ratio according to 1H NMR) of 23 (major isomer, 81%) and2-bromo-5-methylbenzenesulfonyl chloride as a colourless oil. 1H NMR(23) δ 8.20 (d, J = 2.1 Hz, 1H), 7.72 (dd, J = 8.2, 2.0 Hz, 1H), 7.31(dd, J = 8.2, 0.8 Hz, 1H), 2.74 (s, 3H). 13C NMR (23) δ 144.1, 138.2,137.0, 135.0, 131.4, 120.1, 20.0.
References:
Seifert, Tina;Malo, Marcus;Kokkola, Tarja;Stéen, E. Johanna L.;Meinander, Kristian;Wallén, Erik A.A.;Jarho, Elina M.;Luthman, Kristina [Bioorganic and Medicinal Chemistry,2020,vol. 28,# 2,art. no. 115231]
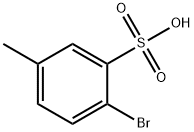
56919-22-3
5 suppliers
inquiry

141113-98-6
20 suppliers
$76.00/250mg

106-38-7
410 suppliers
$14.00/5g

141113-98-6
20 suppliers
$76.00/250mg