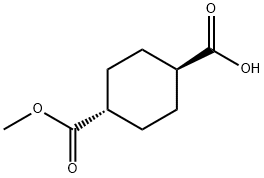
TRANS-1,4-CYCLOHEXANEDICARBOXYLIC ACID MONOMETHYL ESTER synthesis
- Product Name:TRANS-1,4-CYCLOHEXANEDICARBOXYLIC ACID MONOMETHYL ESTER
- CAS Number:15177-67-0
- Molecular formula:C9H14O4
- Molecular Weight:186.21
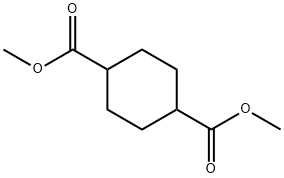
94-60-0

15177-67-0
(1) Isomerization reaction: 0.5 mol (100.1 g) of dimethyl 1,4-cyclohexanedicarboxylate (Ib) with a trans/cis isomer ratio of 3/7 to 5/5 was dissolved in 200 g of methanol at 40°C. The reaction was carried out by adding 15 g of pyridine as a catalyst. 15 g of pyridine was added as a catalyst, and after 2 h of reaction, a methanolic solution of dimethyl 1,4-cyclohexanedicarboxylate (II-b) with a trans/cis isomer ratio of 9/1 was obtained. (2) Monoester hydrolysis reaction: The methanol solution of dimethyl 1,4-cyclohexanedicarboxylate (II-b) with a trans/cis isomer ratio of 9/1 obtained in step (1) was cooled to room temperature, and 150 g of potassium hydroxide was added. The reaction was carried out at 20°C for 3 h. Monoester hydrolysis was carried out to obtain a water-soluble carboxylic acid ester of trans-1,4-cyclohexanedicarboxylic acid monomethyl ester. (3) Acidification and post-treatment: 200 g of water and 100 g of toluene were added to the reaction system of step (2), and the organic phase was removed by extraction and stratification. The aqueous phase was adjusted to pH 1-2 with concentrated hydrochloric acid and the mixture was cooled to 0 °C. After filtration, the mixture was dried under reduced pressure at 50 °C to afford 66.4 g of white solid powder of monomethyl trans-1,4-cyclohexanedicarboxylate (III). The yield was 71.32% and the purity of compound (III) was 98.9% as determined by gas chromatography.

94-60-0
243 suppliers
$6.00/10g

15177-67-0
220 suppliers
$6.00/1g
Yield:15177-67-0 71.32%
Reaction Conditions:
Stage #1:dimethyl 1,4-cyclohexane dicarboxylate with pyridine in methanol at 40; for 2 h;
Stage #2: with potassium hydroxide in methanol at 20; for 3 h;Reagent/catalyst;Temperature;
Steps:
3.1; 3.2; 3.3 A preparation method of trans-1,4-cyclohexanedicarboxylic acid monomethyl ester specifically comprises the following steps:
(1) Isomerization reaction: 0.5 mol, 100.1 g of a compound (I-b) having a trans/cis isomer ratio of 3/7 to 5/5 in a 200 g methanol solution system at a temperature of 40 ° C,After the isomerization reaction under the catalytic condition of 15 g of pyridine for 2 h, a methanol solution of the compound (II-b) having a trans/cis isomer ratio of 9/1 was obtained; (2) Monoester hydrolysis reaction: a methanol solution of the compound (II-b) having a trans/cis isomer ratio of 9/1 in step (1) is cooled to room temperature, and 150 g of potassium hydroxide is added.The hydrolysis temperature is 20 ° C, the reaction time is 3 h, and a water-soluble carboxylate of trans-1,4-cyclohexanedicarboxylic acid monomethyl ester is obtained;(3) Acidification and post-treatment: 200 g of water and 100 g of toluene are added to the reaction system obtained in the step (2), and the organic phase is removed by extraction and layering.The aqueous phase was adjusted to pH 1-2 with concentrated hydrochloric acid, and the temperature was lowered to 0-5 ° C. The mixture was filtered and dried under reduced pressure at 50 ° C to give a white solid powder compound (III) 66.4 g.The yield was 71.32%, and the purity of the compound (III) was determined by gas chromatography to be 98.9%.
References:
He Xia Chemical (Taicang) Co., Ltd.;Bang Tianshenyi;Bang Tianzhizi;Wei Wenjun;Shi Xinghui;Tang Chengjian;Wang Zhengguo CN108129307, 2018, A Location in patent:Paragraph 0027-0041
3399-22-2
156 suppliers
$7.00/1g

15177-67-0
220 suppliers
$6.00/1g
619-82-9
227 suppliers
$5.00/5g

18107-18-1
210 suppliers
$33.00/5g

15177-67-0
220 suppliers
$6.00/1g

619-82-9
227 suppliers
$5.00/5g

15177-67-0
220 suppliers
$6.00/1g
120-61-6
480 suppliers
$6.00/25g

15177-67-0
220 suppliers
$6.00/1g