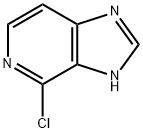
4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE synthesis
- Product Name:4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE
- CAS Number:2770-01-6
- Molecular formula:C6H4ClN3
- Molecular Weight:153.57
![3H-IMidazo[4,5-c]pyridine, 5-oxide](/CAS/GIF/91184-02-0.gif)
91184-02-0
![4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/2770-01-6.gif)
2770-01-6
The general procedure for the synthesis of 4-chloroimidazo[4,5-C]pyridine using the compound (CAS: 91184-02-0) as starting material was as follows: compound 3 (255 mg, 1.88 mmol) was dissolved in 10 mL of phosphorus oxytrichloride (POCl3), and the reaction was stirred for 3 hr at 110 °C until the reaction solution became clear. Upon completion of the reaction, the excess phosphorous trichloride was removed under vacuum. Subsequently, ice water was added to the reaction mixture, the pH was adjusted to about 9 by slowly adding ammonia dropwise, and an equal volume of methanol (MeOH) was added. At this point, a precipitate appeared in the reaction mixture and the precipitate was collected by filtration. The filtrate was adsorbed on silica gel and purified by silica gel column chromatography, the eluent being a mixture of ethyl acetate (EA) and 5% methanol (MeOH), resulting in the target product 4 in 83% yield. The results of high-resolution mass spectrometry (HRMS-ESI) analysis were as follows: m/z calculated value (C6H5ClN3 [M+H]+) was 154.0166, and the measured value was 154.0171.
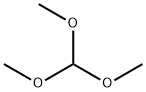
39217-08-8
200 suppliers
$18.00/1g
149-73-5
550 suppliers
$12.00/5g
![4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/2770-01-6.gif)
2770-01-6
135 suppliers
$19.00/250mg
Yield:2770-01-6 98%
Reaction Conditions:
with hydrogenchloride in water at 20;Inert atmosphere;
Steps:
22.1 Step 1: Preparation of 4-chloro-1H-imidazo[4,5-c]pyridine (intermediate S1-1)
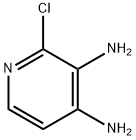
2-chloro-3,4-diaminopyridine (2.87 g, 20 mmol) and trimethyl orthoformate (40 mL) were added into a round-bottom flask, and concentrated hydrochloric acid (3 mL) was slowly added dropwise at room temperature, the mixture solution was stirred overnight at room temperature.
A large number of solids were precipitated and filtered out, and the filter cake was washed with petroleum ether and dried to obtain the crude product of 4-chloro-1H-imidazo[4,5-c]pyridine (3.7 g, yield 98%). MS m/z (ESI): 154 [M+H]+.
References:
Abbisko Therapeutics Co., Ltd.;ZHANG, Mingming;ZHAO, Baowei;YU, Hongping;CHEN, Zhui;XU, Yaochang US2020/140431, 2020, A1 Location in patent:Paragraph 0166; 0211-0213
![3H-IMidazo[4,5-c]pyridine, 5-oxide](/CAS/GIF/91184-02-0.gif)
91184-02-0
16 suppliers
$45.00/2.5mg
![4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/2770-01-6.gif)
2770-01-6
135 suppliers
$19.00/250mg

39217-08-8
200 suppliers
$18.00/1g
14036-06-7
110 suppliers
$56.00/5g
![4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/2770-01-6.gif)
2770-01-6
135 suppliers
$19.00/250mg
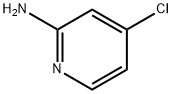
19798-80-2
543 suppliers
$6.00/1g
![4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/2770-01-6.gif)
2770-01-6
135 suppliers
$19.00/250mg

39217-08-8
200 suppliers
$18.00/1g
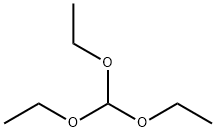
122-51-0
514 suppliers
$10.00/5ml
![4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/2770-01-6.gif)
2770-01-6
135 suppliers
$19.00/250mg