
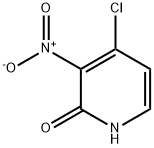
4-Chloro-1-methyl-3-nitro-1H-pyridin-2-one synthesis
- Product Name:4-Chloro-1-methyl-3-nitro-1H-pyridin-2-one
- CAS Number:719268-89-0
- Molecular formula:C6H5ClN2O3
- Molecular Weight:188.57
165547-79-5
133 suppliers
$32.00/1g

74-88-4
359 suppliers
$15.00/10g

719268-89-0
11 suppliers
inquiry
Yield:719268-89-0 94%
Reaction Conditions:
Stage #1: 4-chloro-3-nitropyridin-2(1H)-onewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 20; for 0.5 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide;mineral oil at 20; for 12 h;
Steps:
11 Synthesis of 4-chloro-l-methyI-3-nitropyridin-2(lH)-one
Synthesis of 4-chloro-l-methyI-3-nitropyridin-2(lH)-one ^N^O ΜθΙ/NaH/D F ri^ ^ -NO, Stepl NO, To a stirred solution of 4-chloro-3-nitropyridin-2(l H)-one (3.0 g, 17 mmol) in N,N- dimethyiformarnide (50 mL) was added sodium hydride (60% w/w, 1.0 g, 25.5 mmol) in batches at 0°C. The mixture was stirred at room temperature for 30 minutes. 'Then iodomethane (2.9 g, 20.4 mmol) was added dropwise to the above solution at room temperature .The resultant solution was stirred at room temperature for 12 hours. Once starting material was consumed, the reaction mixture was quenched with ice water (100 mL) at 0~10°C, and extracted with ethyl acetate(100 mL x 3).The organic phase was washed with brine (100 mL x 2), dried over anhydrous sodium sulfate, filtered and concentrated to give a residue, 'The residue was purified by column chromatography (silica gel, dichloromethane/methanol = 10: 1) to give 4-chloro-l- methyl-3- nitropyridin-2( 1 H)-one (3 g, 94%) as a yellow solid. LRMS (M + ΕΓ) m/z; calcd 188.0; found 188.
References:
WO2013/120104,2013,A2 Location in patent:Paragraph 00240