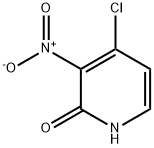
4-Chloro-2-hydroxy-3-nitropyridine synthesis
- Product Name:4-Chloro-2-hydroxy-3-nitropyridine
- CAS Number:165547-79-5
- Molecular formula:C5H3ClN2O3
- Molecular Weight:174.54
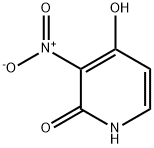
89282-12-2

165547-79-5
General procedure for the synthesis of 2-hydroxy-3-nitro-4-chloropyridine from 4-hydroxy-3-nitropyridin-2(1H)-one: to 2,4-dihydroxy-3-nitropyridine (5.00 g, 0.032 mol), dimethyl chloride (4.07 g, 0.032 mol), and 5 drops of DMF were added to 2,4-dihydroxy-3-nitropyridine (5.00 g, 0.032 mol) and slowly added dropwise to dichloromethane (80 mL) at 0°C. . The reaction mixture was stirred at room temperature for 4 hours. After the reaction was complete, it was diluted by adding dichloromethane (100 mL). The organic layer was washed sequentially with saturated sodium bicarbonate solution, water and saturated saline. The organic layer was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford the target product 2-hydroxy-3-nitro-4-chloropyridine (5.00 g, 89% yield).

89282-12-2
254 suppliers
$8.00/5g

165547-79-5
134 suppliers
$32.00/1g
Yield:-
Reaction Conditions:
with N-benzyl-N,N,N-triethylammonium chloride;trichlorophosphate in water;acetonitrile;
Steps:
68.A Part A.
Part A. 4-chloro-3-nitro-2(1H)-pyridinone: To a solution of 2,4-dihydroxy-3-nitropyridine (5.00 g, 32.03 mmol) and benzyltriethylammonium chloride (29.18 g, 128.12 mmol) in 100 mL of CH3CN was added dropwise phosphorus oxychloride (11.9 mL, 128.12 mmol). The mixture was heated at 60° C. for 1 h and refluxed for 1 h. The solvent was removed and 150 mL of iced water was added. The resulting mixture was stirred at 0° C. for 1.2 h. The precipitate was filtered and dried to give 4.48 g of yellow solid as the desired product. 1H NMR (DMSO-d6) δ 7.79-7.76 (d, 1H), 6.61-6.59 (d, 1H); MS (AP+): 216.0, (M+CH3CN+H)+.
References:
US2002/183324,2002,A1

89282-12-2
254 suppliers
$8.00/5g

165547-79-5
134 suppliers
$32.00/1g