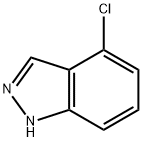
4-CHLORO (1H)INDAZOLE synthesis
- Product Name:4-CHLORO (1H)INDAZOLE
- CAS Number:13096-96-3
- Molecular formula:C7H5ClN2
- Molecular Weight:152.58
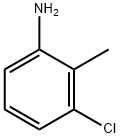
87-60-5

13096-96-3
Step A: Preparation of 4-chloro-1H-indazole: In a 250 mL round-bottomed flask equipped with a stirrer, 2-methyl-3-chloroaniline (8.4 mL, 9.95 g, 70.6 mmol), potassium acetate (8.3 g, 84.7 mmol), and chloroform (120 mL) were added in sequence. The reaction mixture was cooled to 0 °C with stirring. Subsequently, acetic anhydride (20.0 mL, 212 mmol) was added slowly and dropwise over 2 minutes to the cooled mixture. The reaction mixture was gradually warmed to 25 °C and stirred continuously at this temperature for 1 hour. After that, the reaction system was heated to 60 °C and isopentyl nitrite (18.9 mL, 141 mmol) was added. The reaction mixture was stirred at 60 °C overnight. After the reaction was completed, water (75 mL) and THF (150 mL) were added and the mixture was cooled to 0 °C. Next, lithium hydroxide (LiOH, 20.7 g, 494 mmol) was added and the reaction continued to be stirred at 0 °C for 3 hours. After addition of water (200 mL), extraction was performed with ethyl acetate (EtOAc, 300 mL, followed by 100 mL). The organic phases were combined, dried over anhydrous magnesium sulfate (MgSO4) and concentrated under reduced pressure to give 4-chloro-1H-indazole (11.07 g, 100% yield) as an orange solid. The product was confirmed by 1H NMR (400 MHz, CDCl3) and LCMS (ESI pos), 1H NMR data: δ 8.18 (d, J = 1 Hz, 1H), 7.33 (d, J = 8 Hz, 1H), 7.31 (t, J = 7 Hz, 1H), 7.17 (dd, J = 7 Hz, 1 Hz, 1H); LCMS (ESI pos) m/e 153 (M + 1).

87-60-5
450 suppliers
$8.00/25g

13096-96-3
151 suppliers
$15.00/250mg
Yield: 100%
Reaction Conditions:
Stage #1:3-chloro-2-methylbenzenamine with potassium acetate;acetic anhydride in chloroform at 0 - 25; for 1.03333 h;
Stage #2: with isopentyl nitrite in chloroform at 60;
Steps:
10.A.A
Example 10; l-(Tetrahydro-2H-pyran-2-yl)-4-(4,4,5,5-tetramethyl- 1,3,2- dioxaborolan-2-yl)-lH-indazole 62 (Route A)[00252] Step A: Preparation of 4-chloro-lH-indazole: To a 250 ml flask with stirbar was added 2-methyl-3-chloroaniline (8.4 ml, 9.95 g, 70.6 mmol), potassium acetate (8.3 g, 84.7 mmol) and chloroform (120 ml). This mixture was cooled to 0 0C with stirring. To the cooled mixture was added acetic anhydride (20.0 ml, 212 mmol) drop wise over 2 minutes. The reaction mixture was warmed to 25 0C and stirred for 1 hour. At this point, the reaction was heated to 60 0C. Isoamyl nitrite (18.9 ml, 141 mmol) was added and the reaction was stirred overnight at 60 0C. Once complete, water (75 ml) and THF (150 ml) were added and the reaction was cooled to 0 0C. LiOH (20.7 g, 494 mmol) was added and the reaction was stirred at0 0C for 3 hours. Water (200 ml) was added and the product was extracted with EtOAc (300 ml, 100 ml). The organic layers were combined, dried with MgSO4 and concentrated in vacuo to yield 11.07 g 4-chloro-lH-indazole (100%) as an orange solid. 1H NMR (400 MHz, CDCl3) δ 8.18 (d, J= 1 Hz, IH), 7.33 (d, J= 8 Hz IH), 7.31 (t, J= 7 Hz, IH), 7.17 (dd, J= 7 Hz, 1 Hz IH). LCMS (ESI pos) m/e 153 (M+l).
References:
GENENTECH, INC.;F. HOFFMANN - LA ROCHE AG WO2009/42607, 2009, A1 Location in patent:Page/Page column 74
387-45-1
384 suppliers
$9.00/1g

13096-96-3
151 suppliers
$15.00/250mg

387-45-1
384 suppliers
$9.00/1g
443-83-4
246 suppliers
$10.00/25 g

13096-96-3
151 suppliers
$15.00/250mg
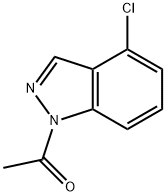
145439-15-2
35 suppliers
$75.00/100mg

13096-96-3
151 suppliers
$15.00/250mg
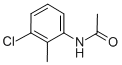
7463-35-6
52 suppliers
$37.00/25g

13096-96-3
151 suppliers
$15.00/250mg