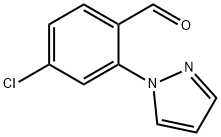
4-Chloro-2-(1H-pyrazol-1-yl)benzaldehyde synthesis
- Product Name:4-Chloro-2-(1H-pyrazol-1-yl)benzaldehyde
- CAS Number:1446818-92-3
- Molecular formula:C10H7ClN2O
- Molecular Weight:206.63
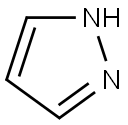
288-13-1
456 suppliers
$10.00/1g
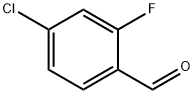
61072-56-8
291 suppliers
$14.00/5g

1446818-92-3
5 suppliers
inquiry
Yield:1446818-92-3 46%
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 120; for 3.5 h;
Steps:
70.1 Step 1: Preparation of 3-chloro-2-(morpholin-4-yl)benzaldehyde
A 100 mL round-bottom flask was charged with 3-chloro-2-fluorobenzaldehyde (3.00 g, 18.9 mmol, 1.00 equiv), morpholine (2.50 g, 28.7 mmol, 1.52 equiv), potassium carbonate (6.50 g, 47.0 mmol, 2.49 equiv), and dimethyl sulfoxide (30 mL). The resulting solution was stirred for 3 hours at 100 °C in an oil bath and diluted with 0 (30 mL). The resulting solution was extracted with dichloromethane (3 x 20 mL) and the organic layers were combined, washed with H20 (2 x 50 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was chromatographed on a silica gel columnwith ethyl acetate/petroleum ether (1/20) to provide 1.40 g (33% yield) of 3-chloro-2- (morpholin-4-yl)benzaldehyde as a yellow solid. LCMS (ESI, m/z): 226 [M+H]+.
References:
WO2013/103973,2013,A1 Location in patent:Paragraph 00252