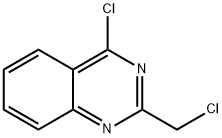
4-CHLORO-2-CHLOROMETHYLQUINAZOLINE synthesis
- Product Name:4-CHLORO-2-CHLOROMETHYLQUINAZOLINE
- CAS Number:34637-41-7
- Molecular formula:C9H6Cl2N2
- Molecular Weight:213.06
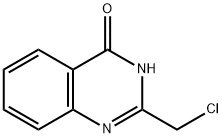
3817-05-8
64 suppliers
$60.00/50mg

34637-41-7
44 suppliers
$180.00/1 G
Yield: 69%
Reaction Conditions:
Stage #1:2-(chloromethyl)quinazolin-4(3H)-one with N-ethyl-N,N-diisopropylamine;trichlorophosphate in toluene at 65;
Stage #2: with sodium hydrogencarbonate in water;toluene
Steps:
4; 45
A suspension of 2-chloromethyl-3H-quinazolin-4-one (12.27 g) in toluene (200 mL) was treated with Hünig's base (19 mL, 109 mmol) and POCl3 (8.8 mL, 96.1 mmol) and heated to 65° C. overnight. The reaction was cooled to rt and the layers separated. The bottom layer was extracted with toluene. The top layers were combined and washed with cold H2O and satd NaHCO3, dried (MgSO4), filtered and concentrated. Purification by gradient MPLC (SiO2, 120 g column, EtOAc/hexanes, 0-100%) provided 9.72 g (69%) of the title compound as a white solid. 1H NMR (DMSO-d6) δ 8.33 (ddd, 1H), 8.05-8.22 (m, 2H), 7.93 (ddd, 1H), 4.97 (s, 2H); LC-MS (ESI+; 213 ([M+H]+).
References:
Myriad Pharmaceuticals, Inc. US2010/68197, 2010, A1 Location in patent:Page/Page column 7; 26

107-14-2
297 suppliers
$15.00/25g
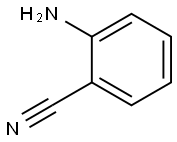
1885-29-6
457 suppliers
$5.00/1g

34637-41-7
44 suppliers
$180.00/1 G

3817-05-8
64 suppliers
$60.00/50mg

34637-41-7
44 suppliers
$180.00/1 G
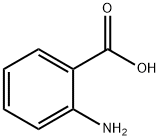
118-92-3
0 suppliers
$21.60/25g

34637-41-7
44 suppliers
$180.00/1 G
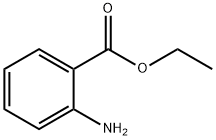
87-25-2
247 suppliers
$18.00/25mL

34637-41-7
44 suppliers
$180.00/1 G