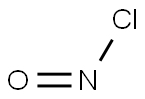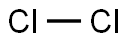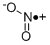
NITROGEN DIOXIDE synthesis
- Product Name:NITROGEN DIOXIDE
- CAS Number:10102-44-0
- Molecular formula:NO2*
- Molecular Weight:46.01
Discoveries on the role forNOin biology and medicine led to a 1998 Nobel Prize for Robert Furchgott, Louis J. Ignarro, and Ferid Murad. Nitric oxide and NO2 occur naturally by bacterial degradation of nitrogenous compounds and to a lesser extent from fires, volcanic action, and fixation by lightning. NO has been the subject of intense and extensive research in a vast array of fields including chemistry, molecular biology, pharmaceuticals, and gene therapy. Formed endogenously, NO has a physiological role in blood flow regulation, thrombosis, and neurotransmission, and a pathophysiological role in inflammation, oxidative stress, and host defense. NO is derived from the amino acid L-arginine by five-electron oxidation catalyzed by NO synthase (requiring reduced pyridine nucleotides, reduced biopteridines, and calmodulin). The by-product, citrulline, is recycled back to L-arginine. In the bloodstream, NO binds primarily hemoglobin, is converted to NO3, and is eliminated in the urine with a half-life of 5–8 h.
Nitric oxide is manufactured by passing air through an electric arc or by oxidation of ammonia over platinum gauze.
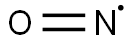
10102-43-9
63 suppliers
$479.00/1Kit
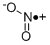
10102-44-0
0 suppliers
inquiry
Yield:10102-44-0 95%
Reaction Conditions:
with oxygen in gasKinetics;1% NO in air; 20--183°C; reaction time 6.6-384s;
References:
Briner, E;Pfeiffer, W.;Malet, G. [Journal de Chimie Physique et de Physico-Chimie Biologique,1924,vol. 21,p. 25 - 29] Schoenherr [Elektrotechnische Zeitschrift,1909,vol. 3,p. 400]
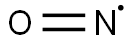
10102-43-9
63 suppliers
$479.00/1Kit

7727-37-9
115 suppliers
$268.00/56l
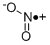
10102-44-0
0 suppliers
inquiry
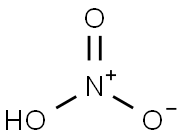
7697-37-2
0 suppliers
$13.00/25g
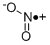
10102-44-0
0 suppliers
inquiry
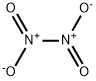
10544-72-6
0 suppliers
inquiry
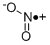
10102-44-0
0 suppliers
inquiry