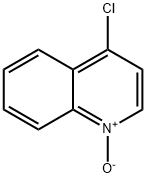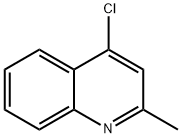
4-CHLORO-2-METHYLQUINOLINE synthesis
- Product Name:4-CHLORO-2-METHYLQUINOLINE
- CAS Number:4295-06-1
- Molecular formula:C10H8ClN
- Molecular Weight:177.63
607-67-0

4295-06-1
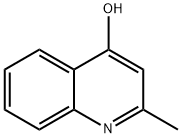
General procedure: 4-hydroxy-2-methylquinoline (5a-c) was added to POCl3 and the reaction mixture was heated to reflux for 12 hours. Upon completion of the reaction, the excess POCl3 was removed by distillation under reduced pressure. the residue was washed once and twice each with CHCl3 and toluene sequentially. The solid obtained was dissolved in CH2Cl2 and triethylamine was added dropwise until the pH of the aqueous wash solution was greater than 10. Finally, the reaction mixture was filtered to afford the target products 2-methyl-4-chloroquinoline (6a-c).

607-67-0
171 suppliers
$10.00/1g

4295-06-1
140 suppliers
$15.00/1g
Yield:4295-06-1 93%
Reaction Conditions:
with trichlorophosphate at 80; for 5 h;
Steps:
1.1
4-chloro-2-methylquinoline (compound d) will be 35mL POCl3A mixture with 5 g of 4-hydroxy-2-methylquinoline was heated to 80 ° C for 5 hours.After cooling to room temperature, the reaction mixture was poured into ice water and neutralized with sodium hydroxide and then extracted with dichloromethane.The combined organic layers were dried over anhydrous sodium sulfate and filtered.Using a concentrated organic layer, a pale yellow purified compound was obtained by using silica gel column chromatography and petroleum ether-ethyl acetate (100:1) as eluent.Yield: 5.18 g (93%).
References:
Chongqing University of Technology;Li Shuo;You Donghui;Tao Chuanyi;Li Na;Wang Mingqi CN109867625, 2019, A Location in patent:Paragraph 0047; 0049-0050; 0052
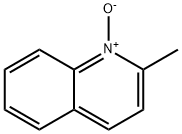
1076-28-4
38 suppliers
$54.00/1g

4295-06-1
140 suppliers
$15.00/1g
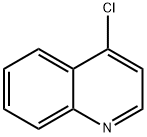
611-35-8
215 suppliers
$5.00/250mg
917-54-4
184 suppliers
$44.39/50ml

4295-06-1
140 suppliers
$15.00/1g

607-67-0
171 suppliers
$10.00/1g
497-19-8
1488 suppliers
$13.00/300G

4295-06-1
140 suppliers
$15.00/1g