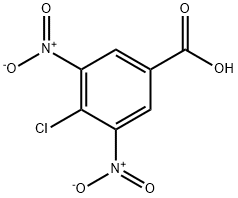
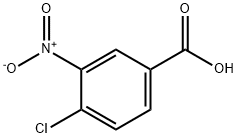
4-Chloro-3,5-dinitrobenzoic acid synthesis
- Product Name:4-Chloro-3,5-dinitrobenzoic acid
- CAS Number:118-97-8
- Molecular formula:C7H3ClN2O6
- Molecular Weight:246.56
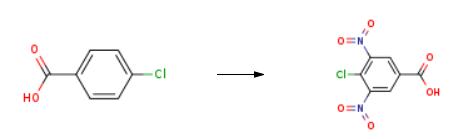
4-chloro-benzoic acid (20 g, 0.128 mol) was dissolved in H2SO4 (d = 1.835 g/mL, 300 mL) at80 C, and KNO3 (66 g, 0.65 mol) was added. The reaction mixture was heated to 125 C using ahigh-pressure flask and kept for 2 h, after which the reaction was cooled to rt and poured onto ice.The yield of 4-Chloro-3,5-dinitrobenzoic acid was 28 g (89%) .
74-11-3
601 suppliers
$5.00/1g

118-97-8
239 suppliers
$15.00/10g
Yield:118-97-8 89%
Reaction Conditions:
with sulfuric acid;potassium nitrate||KNO3||NO3K at 125; for 2 h;High pressure;
Steps:
5.1.1. 4-Chloro-3,5-Dinitrobenzoic Acid (2)
4-chloro-benzoic acid 1 (20 g, 0.128 mol) was dissolved in H2SO4 (d = 1.835 g/mL, 300 mL) at80 C, and KNO3 (66 g, 0.65 mol) was added. The reaction mixture was heated to 125 C using ahigh-pressure flask and kept for 2 h, after which the reaction was cooled to rt and poured onto ice.The yield of title compound 2 was 28 g (89%) [16]. 1H NMR (300 MHz, DMSO-d6) = 14.29 (s, 1H),8.76 (s, 2H). 13C NMR (75 MHz, DMSO-d6) 163.61, 149.06, 132.20, 128.70, 122.80. HRMS (ESI): m/zcalcd. for C7H2ClN2O6 [MH]: 244.9607; found, 244.9607.
References:
Aerschot, Arthur Van;Gadakh, Bharat;Lescrinier, Eveline;Nautiyal, Manesh;Pang, Luping;Rozenski, Jef;Strelkov, Sergei V.;Weeks, Stephen D.;Zhang, Baole;de Graef, Steff [Molecules,2020,vol. 25,# 20,art. no. 4751]
106-43-4
356 suppliers
$14.00/5g

118-97-8
239 suppliers
$15.00/10g
96-99-1
520 suppliers
$5.00/5g

118-97-8
239 suppliers
$15.00/10g
34656-93-4
0 suppliers
inquiry

118-97-8
239 suppliers
$15.00/10g
7221-27-4
31 suppliers
inquiry

118-97-8
239 suppliers
$15.00/10g