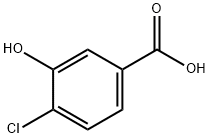
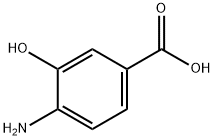
4-Chloro-3-hydroxybenzoic acid synthesis
- Product Name:4-Chloro-3-hydroxybenzoic acid
- CAS Number:34113-69-4
- Molecular formula:C7H5ClO3
- Molecular Weight:172.57

931409-94-8

34113-69-4
General procedure for the synthesis of 4-chloro-3-hydroxybenzoic acid from compound (CAS: 931409-94-8): boron tribromide (1 M in dichloromethane, 31 mL, 31.504 mmol) was slowly added dropwise to a solution of compound 38 (3.56 g, 15.752 mmol) in dichloromethane (30 mL) under ice bath cooling conditions. The reaction mixture was stirred at 0°C to room temperature for 18 hours. Upon completion of the reaction, the reaction was quenched with 0.88 aqueous ammonia solution and stirring was continued for 90 minutes at room temperature. Subsequently, the reaction mixture was acidified to pH 1 by dropwise addition of 2N aqueous hydrochloric acid solution and then extracted with ether (2 x 50 mL). The organic layers were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford 4-chloro-3-hydroxybenzoic acid as a light yellow solid in 90% yield (2.45 g). The product was characterized by 1H NMR (400 MHz, CD3OD): δ 7.36-7.38 (d, 1H), 7.44-7.47 (dd, 1H), 7.54-7.55 (d, 1H); LRMS APCI m/z 171 [M-H]-.
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

34113-69-4
144 suppliers
$6.00/250mg
Yield: 90%
Reaction Conditions:
Stage #1:3-methoxy-4-chlorobenzoic acid allyl ester with boron tribromide in dichloromethane at 0 - 20; for 18 h;
Stage #2: with ammonia in dichloromethane;water at 20; for 1.5 h;
Stage #3: with hydrogenchloride in dichloromethane;water; pH=1
Steps:
39
Boron tribromide (1M in dichloromethane, 31 mL, 31.504mmol) was added to an ice-cooled solution of the product of preparation 38 (3.56g, 15.752mmol) in dichloromethane (3OmL) and the mixture was stirred at O0C to room temperature for 18 hours. The reaction was quenched with 0.88 ammonia solution and stirred at room temperature for 90 minutes. The reaction mixture was acidified to pH 1 by dropwise addition of 2N hydrochloric acid (aq) and extracted with diethyl ether (2x50mL). The combined organic layers were dried over sodium sulfate and concentrated in vacuo to afford the title compound as a pale yellow solid in 90% yield, 2.45g. 1HNMR(400MHz, CD3OD) δ: 7.36-7.38(d, 1H), 7.44-7.47(dd, 1H), 7.54-7.55(d, 1H); LRMS APCI m/z 171 [M-H]-
References:
Location in patent:Page/Page column 53
99-06-9
528 suppliers
$5.00/25g

34113-69-4
144 suppliers
$6.00/250mg

99-06-9
528 suppliers
$5.00/25g
51786-10-8
53 suppliers
$55.00/100mg

34113-69-4
144 suppliers
$6.00/250mg
56961-30-9
150 suppliers
$18.00/1g
2374-03-0
349 suppliers
$12.72/1gm:

34113-69-4
144 suppliers
$6.00/250mg