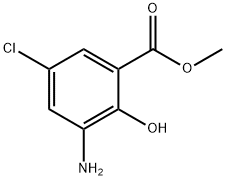
Methyl 3-amino-5-chloro-2-hydroxybenzoate synthesis
- Product Name:Methyl 3-amino-5-chloro-2-hydroxybenzoate
- CAS Number:5043-81-2
- Molecular formula:C8H8ClNO3
- Molecular Weight:201.61
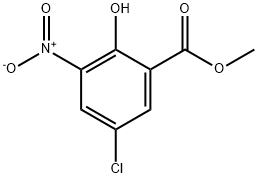
5043-79-8

5043-81-2
General procedure for the synthesis of methyl 3-amino-5-chloro-2-hydroxy-2-hydroxybenzoate from methyl 5-chloro-2-hydroxy-3-nitrobenzoate: Methyl 5-chloro-2-hydroxy-3-nitrobenzoate (2.00 g, 10.7 mmol) was dissolved in 6 mL of concentrated sulfuric acid and stirred at 0 °C. Subsequently, a mixture of concentrated sulfuric acid and concentrated nitric acid (1:1, 1.6 mL, v/v) was slowly added, and the reaction mixture was gradually warmed up to room temperature with continuous stirring for 24 hours. After the reaction was completed, the mixture was poured into ice water, the precipitate was collected by filtration, washed with water and dried to obtain the nitro compound. The nitro compound was suspended in a solvent mixture of 15 mL of ethyl acetate and 15 mL of methanol, 5% palladium/carbon catalyst (0.350 g) was added, and the hydrogenation reduction reaction was carried out at room temperature for 4 hours. At the end of the reaction, the catalyst was removed by filtration and the filtrate was concentrated to afford methyl 3-amino-5-chloro-2-hydroxybenzoate solid 1.61 g in 74.6% yield. The product was characterized by 1H-NMR (CDCl3): δ 10.84 (1H, s), 7.20 (1H, d, J=2.4Hz), 6.83 (1H, d, J=2.4Hz), 3.94 (3H, s).

5043-79-8
64 suppliers
$35.00/1g

5043-81-2
122 suppliers
$31.00/250mg
Yield: 96.3%
Reaction Conditions:
with tin(II) chloride dihdyrate in ethanol at 81; for 1 h;Temperature;Reagent/catalyst;Solvent;
Steps:
1-3; 1-3 Preparation of intermediate 3-amino-5-chloro-2-hydroxybenzoic acid methyl ester
(1) Dissolve 868g 5-chloro-2-hydroxy-3-nitrobenzoic acid methyl ester in 874ml ethanol,Add 4.65kg of stannous chloride hydrate and heat to reflux at 81°C for 1 hour while stirring.Use a glass rod to drop a small amount of reaction liquid on the TLC plate. If the spot is not yellow, it indicates that the reaction is complete;(2) After the reaction is complete, the reaction solution is evaporated to nearly dryness using a rotary evaporator, and ether is added in batches to wash 3 times.After drying under reduced pressure, 728 g of white solid was obtained with a yield of 96.3%.
References:
Shanghai Xinyi Vientiane Pharmaceutical Co., Ltd.;Shanghai Xinyi Yan'an Pharmaceutical Co., Ltd.;Zhang Yawen;Qian Lei;Gao Jing;Dong Jiali;Jiang Lili;Guo Jingjing;Wang Ping;Ye Zhihong;Zhang Chi;Gu Yu CN112062687, 2020, A Location in patent:Paragraph 0022-0055
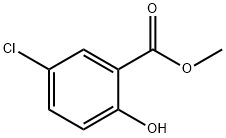
4068-78-4
199 suppliers
$12.00/5g

5043-81-2
122 suppliers
$31.00/250mg