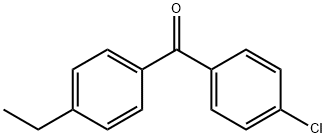
4-CHLORO-4'-ETHYLBENZOPHENONE synthesis
- Product Name:4-CHLORO-4'-ETHYLBENZOPHENONE
- CAS Number:71766-56-8
- Molecular formula:C15H13ClO
- Molecular Weight:244.72
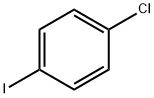
637-87-6
306 suppliers
$38.00/10g
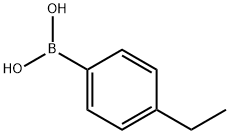
63139-21-9
289 suppliers
$5.00/250mg
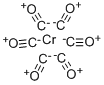
13007-92-6
202 suppliers
$41.70/1g

71766-56-8
19 suppliers
$294.00/1g
Yield:71766-56-8 95%
Reaction Conditions:
with sodium t-butanolate in N,N-dimethyl acetamide at 80; for 0.25 h;Suzuki Coupling;
Steps:
2.6 Typical Procedure for the Carbonylative Suzuki Coupling Reaction in the Presence of SBA-16/DFMP-Pd
General procedure: Aryl halide (1.0 mmol), arylboronic acid (1.1 mmol),t-BuONa (2.0 mmol), SBA-16/DFMP-Pd (0.0026 g,0.2 mol%), and Cr(CO)6 (0.5 mmol, 0.11 g) in DMA(3.0mL) was stirred in a flask at 80°C. The reaction progress was monitored by thin layer chromatography (TLC).Upon the reaction’s completion, the mixture was allowed to cool down to room temperature and then filtered and washed with H2Oand ethyl acetate. The organic fraction was separated,dried over anhydrous Na2SO4 and then the solvent was removed using a rotary evaporator. Further purification was performed on silica gel column chromatography to afford the pure products.
References:
Niakan, Mahsa;Asadi, Zahra;Emami, Mohammad [Catalysis Letters,2020,vol. 150,# 2,p. 404 - 418]

63139-21-9
289 suppliers
$5.00/250mg
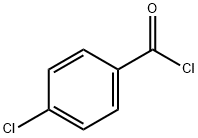
122-01-0
384 suppliers
$13.00/25g

71766-56-8
19 suppliers
$294.00/1g

637-87-6
306 suppliers
$38.00/10g

63139-21-9
289 suppliers
$5.00/250mg
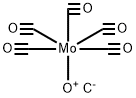
199620-15-0
0 suppliers
inquiry

71766-56-8
19 suppliers
$294.00/1g
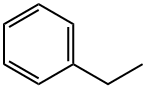
100-41-4
369 suppliers
$5.00/5G

122-01-0
384 suppliers
$13.00/25g

71766-56-8
19 suppliers
$294.00/1g