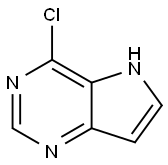
4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE synthesis
- Product Name:4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE
- CAS Number:84905-80-6
- Molecular formula:C6H4ClN3
- Molecular Weight:153.57
![1,5-DIHYDRO-4H-PYRROLO[3,2-D]PYRIMIDIN-4-ONE](/CAS/GIF/5655-01-6.gif)
5655-01-6
![4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE](/CAS/GIF/84905-80-6.gif)
84905-80-6
Step 4: Preparation of 4-chloro-5H-pyrrolo[3,2-d]pyrimidine (32e). 3H,5H-pyrrolo[3,2-d]pyrimidin-4-one (32d) (31.08 g, 230 mmol) was placed under a nitrogen atmosphere and phosphoryl chloride (60 mL, 644 mmol) was added. The reaction mixture was heated to reflux for 1 h, during which the mixture was observed to transform into a black homogeneous solution. Upon completion of the reaction, the mixture was cooled in an ice-water bath and subsequently slowly poured into crushed ice (775 mL) under stirring. The pH of the aqueous solution was slowly adjusted to about 8 with concentrated ammonia (225 mL) while keeping the mixture cool. The resulting precipitate was collected by vacuum filtration and washed with cold water. The solid was transferred to a drying tray and dried under vacuum at 110 °C to afford 4-chloro-5H-pyrrolo[3,2-d]pyrimidine (32e) (31.48 g, 89% yield) as a dark gray solid. To obtain an analytically pure sample, it was purified by silica gel column chromatography (eluent: EtOAc-hexane, 35:65) and the relevant fractions were collected and concentrated. Grinding of the solid with EtOAc-MeOH mixed solvent afforded an off-white solid 4-chloro-5H-pyrrolo[3,2-d]pyrimidine (32e) with a melting point >150 °C (decomposition).1H NMR (DMSO-d6) δ 12.43 (s, D2O exchangeable, 1H), 8.61 (s, 1H), 7.97 (dd, J = 2.8, 2.8 Hz; D2O-exchangeable to d, 1H), 6.72 (dd, J = 1.7, 3.5 Hz; D2O-exchangeable to d, 1H).13C NMR (DMSO-d6) δ 151.30, 149.58, 142.12, 134.83, 124.32, 102.70.IR (pure sample) 3128, 3078, 2979, 1621 cm-1. MS (ES+) m/z 154.01 (100%, [M+H]+), 156.01 (33%). Elemental analysis (C6H4N3Cl) Calculated values: C, 46.93; H, 2.63; N, 27.36; Cl, 23.09. Measured values: C, 47.10; H, 2.79; N, 27.15; Cl, 22.93.
![1,5-DIHYDRO-4H-PYRROLO[3,2-D]PYRIMIDIN-4-ONE](/CAS/GIF/5655-01-6.gif)
5655-01-6
115 suppliers
$45.00/50mg
![4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE](/CAS/GIF/84905-80-6.gif)
84905-80-6
206 suppliers
$9.00/250mg
Yield:84905-80-6 93.2%
Reaction Conditions:
with 1,4-diaza-bicyclo[2.2.2]octane;triethylamine;trichlorophosphate in toluene at 50; for 15 h;Temperature;Reagent/catalyst;Solvent;
Steps:
1.3-8.3 (3) Formula I - Synthesis of 4-chloropyrrolopyrimidine:
Adding 4-hydroxypyrrolopyrimidine to 1000 ml of toluene to form an organic mixed solution of 4-hydroxypyrrolopyrimidine;After adding a chlorinating reagent-triethylenediamine to the organic mixed solution of 4-hydroxypyrrolopyrimidine, the temperature is raised to 50 ° C.Adding organic lye triethylamine,After the completion of the dropwise addition, the temperature was raised to 50 ° C, and the reaction was further concentrated for 15 hours. The concentrate was poured into ice water, the pH was adjusted to 7-8 with sodium carbonate, and the solid was collected by filtration. The solid was dissolved in acetone, decolorized, and recrystallized. White solid 4-chloropyrrolopyrimidine in a yield of 93.2%. The total yield was 77.3% and the purity was greater than 99.1%.
References:
CN109824675,2019,A Location in patent:Page/Page column 7-12
![1,5-DIHYDRO-4H-PYRROLO[3,2-D]PYRIMIDIN-4-ONE](/CAS/GIF/5655-01-6.gif)
5655-01-6
115 suppliers
$45.00/50mg
![4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE](/CAS/GIF/84905-80-6.gif)
84905-80-6
206 suppliers
$9.00/250mg
![ethyl 4-hydroxy-5H-pyrrolo[3,2-d]pyriMidine-7-carboxylate](/CAS/20150408/GIF/779326-74-8.gif)
779326-74-8
7 suppliers
inquiry
![4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE](/CAS/GIF/84905-80-6.gif)
84905-80-6
206 suppliers
$9.00/250mg
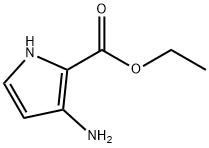
252932-48-2
101 suppliers
$30.00/1g
![4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE](/CAS/GIF/84905-80-6.gif)
84905-80-6
206 suppliers
$9.00/250mg
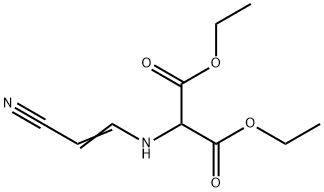
1021175-71-2
19 suppliers
inquiry
![4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE](/CAS/GIF/84905-80-6.gif)
84905-80-6
206 suppliers
$9.00/250mg