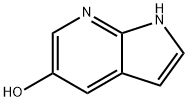
1H-PYRROLO[2,3-B]PYRIDIN-5-OL synthesis
- Product Name:1H-PYRROLO[2,3-B]PYRIDIN-5-OL
- CAS Number:98549-88-3
- Molecular formula:C7H6N2O
- Molecular Weight:134.14
![5-BROMO-1-TRIISOPROPYLSILANYL-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/858116-66-2.gif)
858116-66-2
![1H-PYRROLO[2,3-B]PYRIDIN-5-OL](/CAS/GIF/98549-88-3.gif)
98549-88-3
General procedure for the synthesis of 1H-pyrrolo[2,3-b]pyridin-5-ol from 1-triisopropylsilyl-5-bromo-7-azaindole: To a 50 mL reaction flask at room temperature were added the compound of formula II (4.08 g, 11.5 mmol), copper acetylacetonate (0.15 g, 0.575 mmol), MHPO (0.15 g, 0.575 mmol), lithium hydroxide monohydrate (2.42 g, 57.5 mmol), 18 mL of DMSO, and 4.5 mL of water. Stirring was turned on, the air in the reaction system was replaced with nitrogen, and the reaction temperature was raised to an internal temperature of 100°C. The reaction was maintained at this temperature for 6 h. The reaction was carried out by TLC. The progress of the reaction was monitored by TLC and after confirming that the ingredients were fully reacted, the heating was stopped and the reaction mixture was cooled to room temperature (20-30°C). 100 mL of water was added to the reaction mixture and the pH was adjusted to 6 with 2 N dilute hydrochloric acid, at which point a solid precipitated. Filtering was carried out and the solid was collected. The aqueous phase was extracted with ethyl acetate (50 mL × 3) and the organic phases were combined. The organic phase was washed sequentially with water and saturated aqueous sodium chloride solution each, and then dried over anhydrous sodium sulfate. The solvent was concentrated under reduced pressure to give the compound of formula III. Yield: 1.2 g, yield: 78.5%.
![5-Benzyloxy-1H-pyrrolo[2,3-b]pyridine](/CAS/20180702/GIF/1036007-71-2.gif)
1036007-71-2
4 suppliers
inquiry
![1H-PYRROLO[2,3-B]PYRIDIN-5-OL](/CAS/GIF/98549-88-3.gif)
98549-88-3
301 suppliers
$8.00/250mg
Yield:98549-88-3 95.5%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in methanol;
Steps:
8 Preparation of 5-hydroxy-7-azaindole
Add 22.4g of 5-benzyloxy-7-azaindole to the hydrogenation bottle, dissolve it with methanol, add 1.1g of palladium carbon, replace the air in the hydrogenation bottle with hydrogen, and then add hydrogen. Stop the reaction after the pressure in the bottle does not change The palladium carbon was removed by filtration, and the organic solvent was swirled to obtain a crude product, which was washed with n-heptane to obtain 12.8 g of a refined product with a yield of 95.5%
References:
CN110922404,2020,A Location in patent:Paragraph 0051-0054
![5-METHOXY-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/183208-36-8.gif)
183208-36-8
169 suppliers
$7.00/100mg
![1H-PYRROLO[2,3-B]PYRIDIN-5-OL](/CAS/GIF/98549-88-3.gif)
98549-88-3
301 suppliers
$8.00/250mg
![5-BROMO-1-TRIISOPROPYLSILANYL-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/858116-66-2.gif)
858116-66-2
76 suppliers
$55.00/250mg
![1H-PYRROLO[2,3-B]PYRIDIN-5-OL](/CAS/GIF/98549-88-3.gif)
98549-88-3
301 suppliers
$8.00/250mg
![5-BROMO-PYRROLO[2,3-B]PYRIDINE-1-CARBOXYLICACIDTERT-BUTYLESTER](/CAS/GIF/928653-80-9.gif)
928653-80-9
52 suppliers
$27.00/100mg
![1H-PYRROLO[2,3-B]PYRIDIN-5-OL](/CAS/GIF/98549-88-3.gif)
98549-88-3
301 suppliers
$8.00/250mg
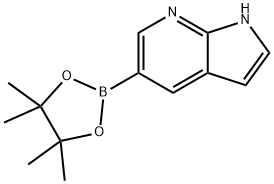
754214-56-7
187 suppliers
$25.00/250mg
![1H-PYRROLO[2,3-B]PYRIDIN-5-OL](/CAS/GIF/98549-88-3.gif)
98549-88-3
301 suppliers
$8.00/250mg