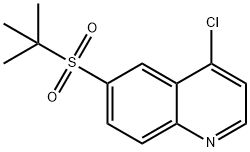
4-chloro-6-[(1,1-diMethylethyl)sulfonyl]quinoline synthesis
- Product Name:4-chloro-6-[(1,1-diMethylethyl)sulfonyl]quinoline
- CAS Number:1346549-11-8
- Molecular formula:C13H14ClNO2S
- Molecular Weight:283.77

1346549-09-4
4 suppliers
inquiry
![4-chloro-6-[(1,1-diMethylethyl)sulfonyl]quinoline](/CAS/20150408/GIF/1346549-11-8.gif)
1346549-11-8
10 suppliers
inquiry
Yield:1346549-11-8 80%
Reaction Conditions:
with Oxone in methanol;water at 20;
Steps:
2.2
4-Chloro-6-[(1,1-dimethylethyl)thio]quinoline (9.4 g, 37 mmol) was suspended in MeOH (100 mL) and water (100 mL) before oxone (25 g, 41 mmol) was added and the reaction was stirred at rt until complete by LCMS (3 hours). The MeOH was removed in vacuo and the heterogeneous aqueous solution was extracted 3* with 100 mL EtOAc. The combined organics were concentrated to provide 8.5 g (80%) of a yellow powder. 1H NMR (DMSO-d6) δ: 9.08 (d, J=4.8 Hz, 1H), 8.63 (d, J=2.0 Hz, 1H), 8.36 (d, J=8.8 Hz, 1H), 8.20 (dd, J=8.8, 2.0 Hz, 1H), 8.00 (d, J=4.8 Hz, 1H), 1.32 (s, 9H); MS (m/z) 284.1 (M+H+).
References:
US2012/41024,2012,A1 Location in patent:Page/Page column 16-17
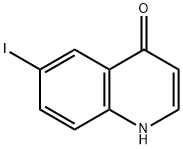
21873-51-8
28 suppliers
$364.00/1g
![4-chloro-6-[(1,1-diMethylethyl)sulfonyl]quinoline](/CAS/20150408/GIF/1346549-11-8.gif)
1346549-11-8
10 suppliers
inquiry
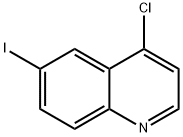
40107-07-1
65 suppliers
$60.00/50mg
![4-chloro-6-[(1,1-diMethylethyl)sulfonyl]quinoline](/CAS/20150408/GIF/1346549-11-8.gif)
1346549-11-8
10 suppliers
inquiry
![5-[[(4-iodophenyl)amino]methylene]-2,2-dimethyl-1,3-dioxane-4,6-dione](/CAS/20180702/GIF/909344-69-0.gif)
909344-69-0
1 suppliers
inquiry
![4-chloro-6-[(1,1-diMethylethyl)sulfonyl]quinoline](/CAS/20150408/GIF/1346549-11-8.gif)
1346549-11-8
10 suppliers
inquiry
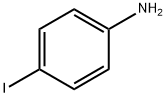
540-37-4
455 suppliers
$6.00/5g
![4-chloro-6-[(1,1-diMethylethyl)sulfonyl]quinoline](/CAS/20150408/GIF/1346549-11-8.gif)
1346549-11-8
10 suppliers
inquiry