
4-Chloro-6-(3,4-dimethoxy-phenyl)-pyrimidine synthesis
- Product Name:4-Chloro-6-(3,4-dimethoxy-phenyl)-pyrimidine
- CAS Number:103555-31-3
- Molecular formula:C12H11ClN2O2
- Molecular Weight:250.6809
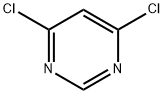
1193-21-1
553 suppliers
$14.00/5g
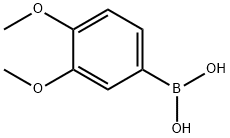
122775-35-3
321 suppliers
$5.00/1g

103555-31-3
4 suppliers
inquiry
Yield:103555-31-3 97.56%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in 1,4-dioxane;water at 90; for 20 h;Inert atmosphere;
Steps:
2.B Example 2 4-Chloro-6-(3,4-dimethoxy-phenyl)-pyrimidine Method B
To 4,6-dichloropyrimidine (0.350 g, 2.36 mmol) in 1 ,4-dioxane (1 0 mL) was added 3,4-dimethoxyphenylboronic acid (0.3 g, 1 .64 mmol) and tetrakis (triphenyl phosphine) palladium (0.06 g, 0.06 mmol). Potassium carbonate (0.44 g, 3.2 mmol) was dissolved in water (5 mL) and was added to the reaction mixture. The reaction mixture was stirred under nitrogen at 90 °C for 20 h. The reaction mixture was cooled to room temperature and was concentrated. The residue was partitioned between ethyl acetate (2 x 1 0 mL) and water (1 0 mL). Combined organic layers were dried over anhydrous sodium sulphate. The solvent was evaporated to obtain the title compound. Yield: 0.4 g (97.56 %); 1 H NMR (300 MHz, CDCI3) : δ 8.9 (s, 1 H, 2.1 Hz), 7.65 (d, 1 H, 0.9 Hz), 7.60 (dd, 1 H, 2.1 & 8.4 Hz), 6.94 (d, 1 H, 8.4Hz), 3.97 (s, 3H), 3.93 (s, 3H); MS (ES+): 251 (M+1 ).
References:
WO2013/175415,2013,A1 Location in patent:Page/Page column 47

1193-21-1
553 suppliers
$14.00/5g
365564-10-9
103 suppliers
$40.77/1G

103555-31-3
4 suppliers
inquiry
91-16-7
546 suppliers
$13.00/25g

103555-31-3
4 suppliers
inquiry