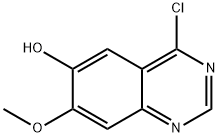
6-Quinazolinol, 4-chloro-7-methoxy- synthesis
- Product Name:6-Quinazolinol, 4-chloro-7-methoxy-
- CAS Number:574745-97-4
- Molecular formula:C9H7ClN2O2
- Molecular Weight:210.62
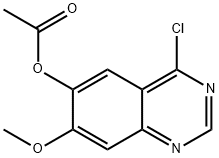
230955-75-6

574745-97-4
General procedure for the synthesis of 4-chloro-7-methoxy-6-ol from 6-acetoxy-4-chloro-7-methoxyquinazoline: 4-chloro-7-methoxyquinazolin-6-yl acetate (prepared according to the method described in Example 25-5 of WO01/66099, 10.1 g, 40 mmol) was suspended in 6 N methanol-ammonia solution (200 mL) and stirred at room temperature for 90 minutes at room temperature. Upon completion of the reaction, the solvent was removed by evaporation under vacuum. Water was added to the residue and the resulting suspension was filtered. The collected solids were washed sequentially with water and ether and subsequently dried at high temperature. Finally, vacuum drying in the presence of phosphorus pentoxide gave 4-chloro-7-methoxyquinazolin-6-ol (7.9 g, 94% yield). The NMR hydrogen spectrum (DMSO-d6) data were as follows: δ 4.02 (s, 3H), 7.40 (s, 1H), 7.43 (s, 1H), 8.81 (s, 1H).

230955-75-6
146 suppliers
$11.00/250mg

574745-97-4
65 suppliers
$45.00/1g
Yield: 94%
Reaction Conditions:
with ammonia in methanol at 20; for 1.5 h;
Steps:
10.4
A suspension of 4-chloro-7-methoxyquinazolin-6-yl acetate (prepared as described in Example 25-5 of WO01/66099, 10.1 g, 40 mmol) in 6N methanolic ammonia (200 ml) was stirred at room temperature for 90 minutes. The solvents were evaporated under vacuum. Water was added and the resulting suspension was filtered. The solid obtained was washed with water, ether and dried under high. vacuum in the presence-of phosphorus pentoxide to GIVE 4-CHLORO-7-METHOXYQUINAZOLIN-6-OL (7.9 G, 94%). NMR SPECTRUM: (DMSOD6) 4.02 (S, 3H), 7.40 (s, 1H), 7.43 (s, 1H), 8. 81 (s, 1H).
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED WO2005/26151, 2005, A1 Location in patent:Page/Page column 124
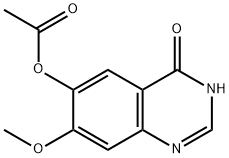
179688-53-0
269 suppliers
$6.00/1g

574745-97-4
65 suppliers
$45.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

574745-97-4
65 suppliers
$45.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
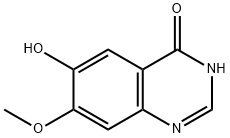
179688-52-9
240 suppliers
$6.00/1g

574745-97-4
65 suppliers
$45.00/1g
6702-50-7
204 suppliers
$11.00/1g

574745-97-4
65 suppliers
$45.00/1g