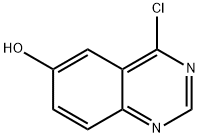
4-CHLORO-6-HYDROXYQUINAZOLINE synthesis
- Product Name:4-CHLORO-6-HYDROXYQUINAZOLINE
- CAS Number:848438-50-6
- Molecular formula:C8H5ClN2O
- Molecular Weight:180.59
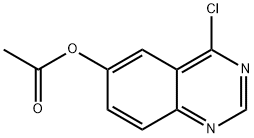
179246-11-8

848438-50-6
Step E: Preparation of 4-chloroquinazolin-6-ol: 4-chloroquinazolin-6-yl acetate (10.0 g, 44.9 mmol) was dissolved in a methanol solution of ammonia (200 mL, 7N), and the reaction was stirred for 1 hour at room temperature. Upon completion of the reaction, the reaction mixture was concentrated under reduced pressure to about 3 mL, and the residue was ground with ether to precipitate a tan solid which was collected by filtration and dried to give 4-chloroquinazolin-6-ol (6.50 g, 80% yield).

179246-11-8
26 suppliers
inquiry

848438-50-6
44 suppliers
$220.00/50mg
Yield:848438-50-6 95%
Reaction Conditions:
with ammonia in methanol at 20; for 3 h;Inert atmosphere;
Steps:
The fourth step: the synthesis of 4-chloroquinazolin-6-ol
Dissolve 4-chloroquinazolin-6-yl acetate (700 mg, 3.144 mmol) in methanol (5 mL), add 7N ammonia methanol solution (15 mL), stir at room temperature for 3 hours, add methyl tert-butyl ether, and precipitate The product was precipitated, filtered, and the filter cake was washed with an appropriate amount of methyl tert-butyl ether, and dried to obtain 4-chloroquinazolin-6-ol (600 mg, yield: 95%).
References:
WO2022/105908,2022,A1 Location in patent:Page/Page column 24; 38
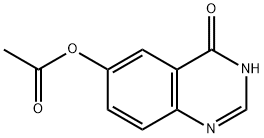
179688-15-4
13 suppliers
inquiry

848438-50-6
44 suppliers
$220.00/50mg
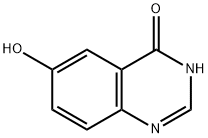
16064-10-1
96 suppliers
$87.00/1g

848438-50-6
44 suppliers
$220.00/50mg
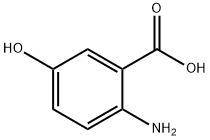
394-31-0
282 suppliers
$7.00/5g

848438-50-6
44 suppliers
$220.00/50mg

16064-10-1
96 suppliers
$87.00/1g

848438-50-6
44 suppliers
$220.00/50mg