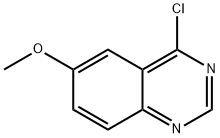
6-METHOXY-QUINAZOLIN-4-YLAMINE synthesis
- Product Name:6-METHOXY-QUINAZOLIN-4-YLAMINE
- CAS Number:50424-28-7
- Molecular formula:C9H7ClN2O
- Molecular Weight:194.62
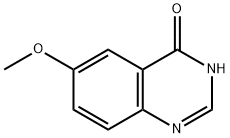
19181-64-7

50424-28-7
Step 2. Synthesis of 4-chloro-6-methoxyquinazoline 0.852 mmol of 6-methoxy-4(1H)-quinazolinone was mixed with 1.022 mmol of phosphorus pentachloride (PCl5) in 0.4 M solution of dichloroethane (DCE). The reaction mixture was reacted in a microwave reactor at 150°C for 400 seconds. After completion of the reaction, the mixture was diluted with dichloromethane (CH2Cl2) and washed sequentially with water and saturated saline. The organic layer was separated, dried over anhydrous magnesium sulfate (MgSO4), filtered and the excess solvent was removed by rotary evaporator to afford 4-chloro-6-methoxyquinazoline in 75% yield. The product was used directly in the subsequent reaction without further purification. m/z = 195 ([M+H]+) was shown by LC-MS analysis.

19181-64-7
87 suppliers
$43.00/250mg

50424-28-7
105 suppliers
$45.00/100mg
Yield: 91%
Reaction Conditions:
with trichlorophosphate at 120;
Steps:
1.2 Step 2: Synthesis of 4- Chloro-6-methoxyquinazoline
[0145] A solution of 6-methoxyquinazolin-4(3H)-one (3.0 g, 17.0 mmol) in POC13 (30 mL) was stirred at 120 °C overnight. Then the mixture was cooled to room temperature and evaporated. The residue was purified by silica column chromatography (PE/EtOAc from 10:1 to 5:1, v/v) to afford 4-chloro-6-methoxyquinazoline (3.0 g, 91% yield).
References:
ABM THERAPEUTICS, INC.;CHEN, Chen WO2019/60611, 2019, A1 Location in patent:Paragraph 0145

19181-64-7
87 suppliers
$43.00/250mg

50424-28-7
105 suppliers
$45.00/100mg
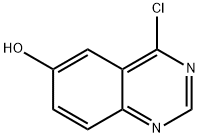
848438-50-6
43 suppliers
$220.00/50mg

74-88-4
356 suppliers
$15.00/10g

50424-28-7
105 suppliers
$45.00/100mg
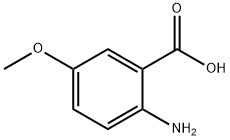
6705-03-9
274 suppliers
$9.00/1g

50424-28-7
105 suppliers
$45.00/100mg
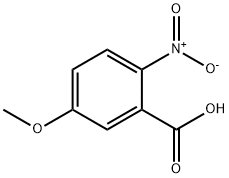
1882-69-5
290 suppliers
$6.00/5g

50424-28-7
105 suppliers
$45.00/100mg