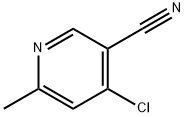
4-CHLORO-6-METHYLNICOTINONITRILE synthesis
- Product Name:4-CHLORO-6-METHYLNICOTINONITRILE
- CAS Number:38875-76-2
- Molecular formula:C7H5ClN2
- Molecular Weight:152.58

1073160-08-3

38875-76-2
Step 8. Preparation of 4-chloro-6-methylnicotinonitrile (B17-8): B17-7 (20 g, 131.5 mmol) was suspended in phosphorus trichloride (POCl3, 62 mL, 580 mmol) and heated at 110 °C for 15 minutes. After the reaction mixture was cooled to 25 °C, phosphorus pentachloride (PCl5, 38.12 g, 183.4 mmol) was added in batches over 20 min. Subsequently, the mixture was heated at 110 °C for 1 h and then concentrated. The resulting residue was diluted with ethyl acetate (EtOAc, 100 mL), cooled to 10 °C and quenched with sodium carbonate (Na2CO3, 200 mL, aqueous solution). The mixture was extracted with ethyl acetate (3 x 250 mL), the organic layers were combined, washed with brine, dried over anhydrous sodium sulfate, and concentrated. The residue was purified by silica gel column chromatography using a petroleum ether solution of 4-5% ethyl acetate as eluent to give B17-8 as an off-white fluffy solid. Yield: 7.5 g, 37%. 1H NMR (CDCl3): δ 8.75 (s, 1H), 7.38 (s, 1H), 2.65 (s, 3H). Mass spectrum: (M + 1) 153, calculated value C7H5ClN2.
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

38875-76-2
66 suppliers
inquiry
Yield: 37%
Reaction Conditions:
Stage #1:6-methyl-4-oxo-1,4-dihydro-pyridine-3-carboxylic acid amide with trichlorophosphate at 110; for 0.25 h;
Stage #2: with phosphorus pentachloride at 20 - 110; for 1.33333 h;
Steps:
8
Step 8. Preparation of 4-Chloro-6-methyl-nicotinonitrile (B17-8): A suspension of B17-7 (20 g, 131.5 mmol) in POCl3 (62 mL, 580 mmol) was heated at 110° C. for 15 minutes. The mixture was allowed to cool to 25° C. and treated portion-wise over 20 minutes with PCl5 (38.12 g, 183.4 mmol). The mixture was then heated at 110° C. for 1 hour and concentrated. The resultant residue was diluted with EtOAc (100 mL), cooled to 10° C., and quenched with aqueous Na2CO3 (200 mL). The mixture was then extracted with EtOAc (3×250 mL), and the combined organic layers were washed with brine, dried over anhydrous sodium sulfate, and concentrated. The resultant residue was purified by chromatography (silica gel; 4-5% EtOAc in petroleum ether as eluting solvent) to provide B17-8 as an off-white puffy solid. Yield: 7.5 g, 37%. 1H NMR (CDCl3): δ 8.75 (s, 1H), 7.38 (s, 1H), 2.65 (s, 3H). Mass: (M+1) 153 calculated for C7H5ClN2.
References:
Pfizer Inc. US2009/54395, 2009, A1 Location in patent:Page/Page column 44
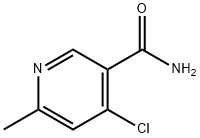
473255-51-5
10 suppliers
inquiry

38875-76-2
66 suppliers
inquiry
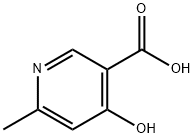
67367-33-3
208 suppliers
$6.00/5g

38875-76-2
66 suppliers
inquiry