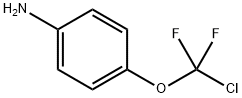
4-(CHLORO-DIFLUORO-METHOXY)-PHENYLAMINE synthesis
- Product Name:4-(CHLORO-DIFLUORO-METHOXY)-PHENYLAMINE
- CAS Number:39065-95-7
- Molecular formula:C7H6ClF2NO
- Molecular Weight:193.58
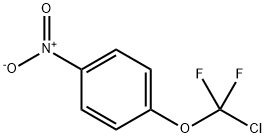
40750-71-8

39065-95-7
General procedure for the synthesis of 4-(chlorodifluoromethoxy)aniline from 1-(chlorodifluoromethoxy)-4-nitrobenzene: 167 g of 1-(chlorodifluoromethoxy)-4-nitrobenzene was added to an autoclave, followed by the addition of 200 g of ethyl acetate and 15 g of nickel Nguyenne. After changing to a nitrogen atmosphere, hydrogen was introduced and the pressure in the autoclave was maintained at 2~3 MPa, and the reaction temperature was controlled at 30-40 °C. After the system pressure was stabilized and no longer decreased, the reaction was continued for 1 hour. Upon completion of the reaction, samples were taken and analyzed to ensure that the residual nitro compounds in the hydrogenation reaction solution were less than 0.5%. Subsequently, the catalyst was removed by filtration, and most of the solvent was firstly recovered by distillation at atmospheric pressure, and then purified by decompression distillation, finally 92.6 g of 4-(chlorodifluoromethoxy)aniline was obtained with a yield of 64.2%.

40750-71-8
52 suppliers
$55.00/50mg

39065-95-7
149 suppliers
$15.00/250mg
Yield:39065-95-7 64.2%
Reaction Conditions:
with hydrogen in ethyl acetate at 30 - 40; under 15001.5 - 22502.3 Torr; for 1 h;Autoclave;Temperature;Solvent;
Steps:
5 Example 5:
167 g of 4-nitro-chlorodifluoromethoxybenzene was charged into the autoclave followed by 200 g of ethyl acetate and 15 g of Raney nickel. After the replacement of nitrogen and hydrogen, the pressure in the autoclave is kept at about 2~3 MPa with hydrogen and reacted at 30-40 ° C. When the pressure of the system is no longer changing, the reaction is maintained for 1 hour. The sample is analyzed and controlled to be less than 0.5% Hydrogenation reaction liquid filtered catalyst, the first atmospheric pressure recovery of most of the solvent, and then vacuum distillation, the target product 4- (chlorodifluoromethoxy) aniline 92.6g, a yield of 64.2%.
References:
CN104119238,2016,B Location in patent:Paragraph 0018; 0032; 0033; 0037; 0038
34888-05-6
28 suppliers
inquiry

39065-95-7
149 suppliers
$15.00/250mg