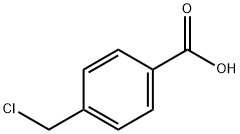
4-(Chloromethyl)benzoic acid synthesis
- Product Name:4-(Chloromethyl)benzoic acid
- CAS Number:1642-81-5
- Molecular formula:C8H7ClO2
- Molecular Weight:170.59
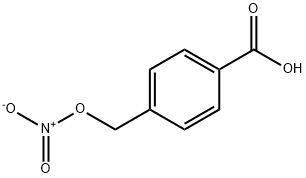
258278-55-6

1642-81-5
Example 1: Synthesis of 4-nitro-oxo-methyl-benzoic acid (I) a) Nitration of 4-chloromethyl-benzoic acid (III) 49.29 kg of 4-chloromethyl-benzoic acid (III) was dissolved in 92.9 L of acetonitrile and stirred for 20 minutes while a slow stream of nitrogen was passed. Subsequently, 93 mL of sulfuric acid was added and stirring was continued for 15 minutes. Under the same operating conditions, 13.65 kg of silver nitrate was slowly added. The reactor was protected from light and the reaction mixture was stirred for 15 minutes. After that, the mixture was heated to reflux for 7 hours and 15 minutes. After completion of the reaction, it was cooled to 20°C-25°C. b) Post-treatment 37.2 L of dimethylformamide (DMF) was added and stirred for 30 min, maintaining the temperature between 20°C-25°C. The silver salt was separated by filtration under nitrogen pressure through a filter pre-washed with 11 L of water and containing 9 kg of filter media. The filter cake was washed with 28 L DMF in three passes. The solid waste was washed twice with 9.3 L DMF. The cellulose in the filter was removed and washed with DMF until clarified, then rinsed with water. c) Precipitation and purification The liquid phase was combined and stabilized at a temperature between 20°C and 25°C. Slowly add 1486 L of water over a period of 1 hour, maintaining the temperature between 20°C-25°C. Continue stirring for 1 hour, maintaining the same temperature range. The precipitate was separated by filtration and the filter cake was washed with water until the pH of the eluate was similar to that of water. Finally, the filter cake was washed with 18.6 L of ethanol. The wet solid was dried under vacuum at not more than 40 °C until the moisture content (KF method) was ≤ 0.2%. 9.68 kg of 4-nitro-oxo-methyl-benzoic acid (I) was obtained in 90.2% yield, 99.35% HPLC purity, and 0.23% (IV).

258278-55-6
0 suppliers
inquiry

1642-81-5
391 suppliers
$17.00/5g
Yield:1642-81-5 90.2%
Reaction Conditions:
with silver nitrate;sulfuric acid in acetonitrile for 7.25 h;Inert atmosphere;Industry scale;Darkness;Reflux;
Steps:
1
Example 1: Synthesis of 4-nitro-oxy-methyl-benzoic acid (I); a) Reaction of 4-chloromethyl-benzoic acid (III) with AqNO and in the presence of H?SC>49.29 kg of 4-chloromethyl-benzoic acid (III) were added to 92.9 I of acetonitrile with stirring for 20 minutes, under a slow nitrogen current. 93 ml of sulphuric acid were added and the mixture was stirred for 15 minutes. 13.65 kg of silver nitrate were added, following the same operation conditions as when adding (III). The reactor was protected from direct exposure to light and the mixture was stirred for 15 minutes.The mixture was then refluxed for 7 hours and 15 minutes. The reaction mix was cooled down to 20°C-25°C. 37.2 I of dimethylformamide were added and it was stirred for 30 minutes, keeping the temperature between 25°C and 20°C.b) Separation of the silver salts by filtrationThe silver salts were separated by filtration, under nitrogen pressure, through a filter containing 9 kg of cellulose, previously washed with 1 1 1 I of water and three times with 28 I of dimethylformamide. The separated solid waste was washed twice with 9.3 I of dimethylformamide. The cellulose was withdrawn from the filter and washed with dimethylformamide until running clear and it was then rinsed with water,c) Precipitation with water The liquid phases were put together and the temperature was stabilised to between 25°C and 20°C. 1486 I of water were added for 1 hour, maintaining the temperature between 20°C and 25°C. The mixture was stirred for 1 hour, maintaining the temperature between 20°C and 25°C. The precipitate was separated by filtration, and the cake thus obtained was washed with water until obtaining a pH similar to that of the water. The cake was finally washed with 18.6 I of ethanol.ngThe wet solid was dried at a temperature of not more than 40°C in a vacuum until the KF water content was of 0.2% at the most. 9.68 kg of4-nitro-oxy-methyl-benzoic acid (I) were obtained. Yield 90.2%. HPLC Purity 99.35%. Content of (IV) 0.23%.
References:
FERRER INTERNACIONAL, S.A.;NICOX S. A.;ANGLADA, Luis;PALOMER, Albert;SOBRAL, Luis WO2011/58162, 2011, A1 Location in patent:Page/Page column 7-8
99-94-5
610 suppliers
$9.00/1g

1642-81-5
391 suppliers
$17.00/5g
3006-96-0
301 suppliers
$14.00/1g

1642-81-5
391 suppliers
$17.00/5g
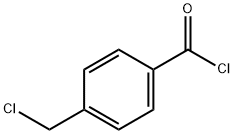
876-08-4
268 suppliers
$11.00/25g

1642-81-5
391 suppliers
$17.00/5g
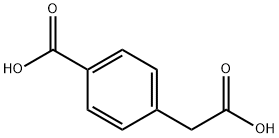
501-89-3
138 suppliers
$7.00/1g

1642-81-5
391 suppliers
$17.00/5g