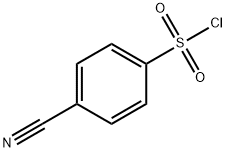
4-CYANOBENZENESULFONYL CHLORIDE 97 synthesis
- Product Name:4-CYANOBENZENESULFONYL CHLORIDE 97
- CAS Number:49584-26-1
- Molecular formula:C7H4ClNO2S
- Molecular Weight:201.63
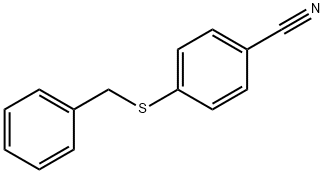
150993-53-6

49584-26-1
N-Chlorosuccinimide (NCS, 1.778 g, 13.32 mmol) was slowly added to a suspension of 4-(benzylthio)benzonitrile (1 g, 4.44 mmol) dissolved in acetic acid (AcOH, 10 mL) and water (3.5 mL) at 0 °C. The reaction mixture was stirred at room temperature for 2 hours. After completion of the reaction, the reaction mixture was diluted with ethyl acetate (EtOAc), washed sequentially with water and saturated brine, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using a hexane solution of 15%-20% ethyl acetate as eluent to give a white solid. The resulting solid was washed with hexane and dried under reduced pressure to give 4-cyanobenzenesulfonyl chloride (0.701 g, 3.48 mmol, 78% yield) as a white solid. The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3): δ 7.91-7.97 (2H, m), 8.15-8.21 (2H, m).
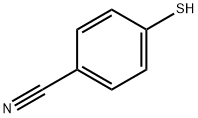
36801-01-1
78 suppliers
inquiry

49584-26-1
170 suppliers
$21.21/1gm:
Yield:49584-26-1 99%
Reaction Conditions:
with N-chloro-succinimide;isopropyl alcohol in dichloromethane at 0 - 20; for 1 h;
Steps:
General procedure for the preparation of sulfonyl halides from thiols
General procedure: To a stirred solution of the corresponding thiol 1 (1 eq) [1]a and isopropanol (CAS: 67-63-0) (2 eq) in dichloromethane [2] (0.15 M), N-bromosuccinimide (CAS: 128-08-5) or N-chlorosuccinimide (CAS: 128-09-6) (4 or 3.5 equiv) was added portion wise1b at rt (in the case of NBS) or 0 °C (in the case of NCS). The reaction mixture is stirred at rt until starting material was not visible by TLC (approx. 1 h). Then, the mixture was diluted with cold, saturated NaHCO3, and extracted with EtOAc (x4).1c The combined organic extracts were dried over anh. Na2SO4 and concentrated in vacuo to afford the crude material. Filtration on a SiO2 column or radial chromatography using mixtures of hexanes/ethyl acetate/acetone as eluents, yielded the pure sulfonyl halides 2 or 3.
References:
Silva-Cuevas, Carolina;Perez-Arrieta, Carlos;Polindara-García, Luis A.;Lujan-Montelongo, J. Armando [Tetrahedron Letters,2017,vol. 58,# 23,p. 2244 - 2247] Location in patent:supporting information

150993-53-6
6 suppliers
inquiry

49584-26-1
170 suppliers
$21.21/1gm:
138-41-0
309 suppliers
$11.00/25g

49584-26-1
170 suppliers
$21.21/1gm:
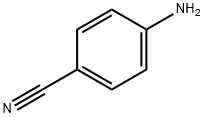
873-74-5
657 suppliers
$9.00/1g

49584-26-1
170 suppliers
$21.21/1gm:
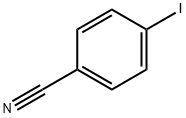
3058-39-7
283 suppliers
$10.00/1g

49584-26-1
170 suppliers
$21.21/1gm: