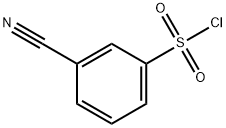
3-Cyanobenzene-1-sulfonyl chloride synthesis
- Product Name:3-Cyanobenzene-1-sulfonyl chloride
- CAS Number:56542-67-7
- Molecular formula:C7H4ClNO2S
- Molecular Weight:201.63
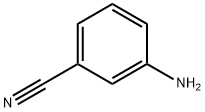
2237-30-1

56542-67-7
General procedure for the synthesis of 3-cyanobenzenesulfonyl chloride from 3-aminobenzonitrile: 3-aminobenzonitrile (2.5 g, 21 mmol) was dissolved in concentrated hydrochloric acid. The mixture of hydrochloric acid (20 mL) and water (20 mL) was cooled to 0 °C and a solution of sodium nitrite (1.5 g, 22 mmol) in water (5 mL) was added slowly and dropwise. The reaction mixture was stirred at 0 °C for 10 min to ensure complete formation of the diazonium salt. In another flask, copper (I) chloride (0.2 g) was added to a solution of acetic acid (25 mL) pre-saturated with sulfur dioxide and stirred at 0°C for 10 minutes. This solution was slowly added dropwise to the above diazonium salt solution, kept at 0°C and stirred for 1 hour. After completion of the reaction, the mixture was poured into ice water and the product was extracted with tert-butyl methyl ether. The organic layers were combined and washed sequentially with water and brine. The crude product was purified by column chromatography (silica gel 60-120 mesh, eluent 5% ethyl acetate in petroleum ether solution) to give pure 3-cyanobenzenesulfonyl chloride (1.9 g, 45% yield) as an off-white solid.1H NMR (300 MHz, CDCl3) δ 8.35 (t, J=1.5 Hz, 1H), 8.31-8.27 (m, 1H), and 8.06-8.02 (m, 1H), 7.82 (t, J=7.9Hz, 1H).

2237-30-1
278 suppliers
$13.00/25g

56542-67-7
160 suppliers
$14.00/1g
Yield:56542-67-7 45%
Reaction Conditions:
Stage #1:m-cyanoaniline with hydrogenchloride;sodium nitrite in water at 0;
Stage #2: with sulfur dioxide;copper(l) chloride in water;acetic acid at 0; for 1.17 h;
Steps:
32 EXAMPLE 32 3-Cyanobenzene-1-sulfonyl chloride
3-Aminobenzonitrile (2.5g, 21 mmol) was dissolved in conc. HCl (20 mL) and water (20 mL), cooled to 0 °C and a solution of sodium nitrite (1.5 g, 22 mmol) in water (5 mL) was added dropwise.
The reaction mixture was stirred for 10 min to complete the diazonium salt formation.
In a separate flask was added copper(I) chloride (0.2g) over a saturated solution of sulfur dioxide in AcOH (25 mL) and stirred at 0 °C for 10 min.
The resulting solution was added dropwise to the diazonium salt and stirred at 0 °C for 1 h.
The reaction mixture poured into ice water and the product was extracted with tert-butylmethylether.
The combined organic layer was washed with water and brine.
The crude product was purified by column chromatography (silica gel 60-120 mesh using 5% EtOAc in petroleum ether) to get the pure 3-cyanobenzene-1-sulfonyl chloride (1.9 g, yield 45%) as an off-white solid. 1H NMR (300MHz, CDCl3) δ 8.35 (t, J = 1.5 Hz, 1 H), 8.31-8.27 (m, 1 H), 8.06-8.02 (m, 1 H), 7.82 (t, J = 7.9 Hz, 1 H).
References:
Tempero Pharmaceuticals, Inc.;BALOGLU, Erkan;GHOSH, Shomir;LOBERA, Mercedes;SCHMIDT, Darby EP2533783, 2015, B1 Location in patent:Paragraph 0335-0336
636-76-0
91 suppliers
$10.00/100mg

56542-67-7
160 suppliers
$14.00/1g
4025-64-3
215 suppliers
$6.00/1g

56542-67-7
160 suppliers
$14.00/1g