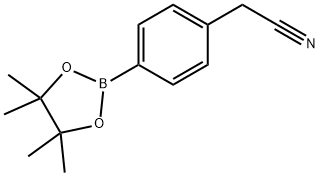
4-(Cyanomethyl)benzeneboronic acid pinacol ester synthesis
- Product Name:4-(Cyanomethyl)benzeneboronic acid pinacol ester
- CAS Number:138500-86-4
- Molecular formula:C14H18BNO2
- Molecular Weight:243.11
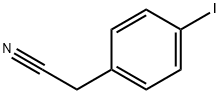
51628-12-7
121 suppliers
$10.00/250mg
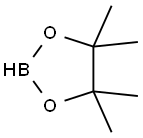
25015-63-8
216 suppliers
$22.39/5G

138500-86-4
116 suppliers
$27.00/1g
Yield:138500-86-4 96.2%
Reaction Conditions:
with triethylamine;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in acetonitrile for 3 h;Heating / reflux;
Steps:
26 Preparation of 4-(cyanomethyl-phenylboronic acid, pinacol ester.
EXAMPLE 26 Preparation of 2-{4-[4-(1-Methyl-2-{[(methylethyl)sulfonyl]amino}ethoxy)phenyl]phenyl}ethanenitrile. Preparation of 4-(cyanomethyl-phenylboronic acid, pinacol ester. Using the method of Murata, M., et al., Y. J. Org. Chem, 62 6458-6459 (1997), 4-iodophenylacetonitrile (23.9 g, 0.100 mol), Et3N (42 ML, 0.30 mol), acetonitrile (400 ML) and Pd(dppf)2Cl2 catalyst ([1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with CH2Cl2) were combined in a 1 L flask and the resulting solution was evacuated and purged with nitrogen three times.The pinacolborane (22 ML, 0.15 mol) was added and the mixture was heated at reflux for 3 h. 1H NMR analysis of an aliquot indicated complete consumption of starting 4-iodophenylacetonitrile.The mixture was cooled to room temperature, concentrated to an oil and taken up in CH2Cl2.This solution was extracted with 0.1 N HCl (3*100 ML) and the organic phase was separated, concentrated and re-dissolved in methyl tert-butyl ether (MTBE).The MTBE solution was passed through a filter packed with silica gel (300 g).The eluant was concentrated to a dark red oil.This oil was extracted with hexanes (500 ML) and the soluble fraction was decanted away from a black oil (5 g). MTBE (5 ML) was added to this oil to give a suspension that was filtered through Celite.This filtrated was combined with the hexanes fraction and the solution was concentrated to an oil.Finally, the oil was re-dissolved in di-isopropyl ether (150 ML) and was diluted with hexanes (400 ML).This mixture was allowed to stand for 2 h, and then filtered to remove a dark precipitate.The resulting amber filtrate was concentrated to an oil, re-dissolved in hexanes (400 ML) and concentrated to 75 ML without added heat.This treatment resulted in the precipitation of the desired intermediate title compound as a golden waxy solid that was air-dried to afford 23.0 g (96.2%): 1H NMR (CDCl3, 300 MHz): δ7.8 (d, 2H); 7.35 (d, 2H); 3.8 (s, 2 H) 1.15 (s, 12H).
References:
Davison, Joshua Zwick;Jones, Winton Dennis;Zarrinmayeh, Hamideh;Zimmerman, Dennis Michael US2003/225266, 2003, A1 Location in patent:Page 23
![Benzeneacetonitrile, 4-[(phenylsulfonyl)oxy]-](/CAS/20200611/GIF/1350764-44-1.gif)
1350764-44-1
0 suppliers
inquiry

25015-63-8
216 suppliers
$22.39/5G

138500-86-4
116 suppliers
$27.00/1g
14191-95-8
413 suppliers
$6.00/5g

138500-86-4
116 suppliers
$27.00/1g
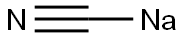
143-33-9
0 suppliers
$10.00/1g
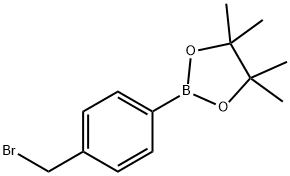
138500-85-3
268 suppliers
$8.00/1g

138500-86-4
116 suppliers
$27.00/1g
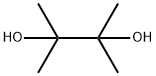
76-09-5
397 suppliers
$8.19/250mg

138500-86-4
116 suppliers
$27.00/1g