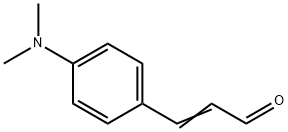
4-(Dimethylamino)cinnamaldehyde synthesis
- Product Name:4-(Dimethylamino)cinnamaldehyde
- CAS Number:6203-18-5
- Molecular formula:C11H13NO
- Molecular Weight:175.23

75-07-0
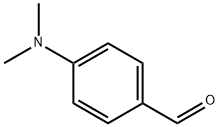
100-10-7
![3-[4-(dimethylamino)phenyl]prop-2-enal](/CAS/20210111/GIF/20432-35-3.gif)
20432-35-3
General procedure for the synthesis of (E)-3-(4-(dimethylamino)phenyl)acrolein from acetaldehyde and p-dimethylaminobenzaldehyde: A mixture of 4-N,N-dimethylaminobenzaldehyde (5.00 g, 34 mmol) with 25 ml of concentrated sulfuric acid was cooled to 0°C. Subsequently, 1 ml of distilled water was slowly added. Keeping the reaction mixture at 0°C, acetaldehyde (5.6 ml, 102 mmol) was added dropwise, ensuring that the reaction temperature did not exceed 2°C. After the dropwise addition, the dark reaction mixture was continued to be stirred at 0°C for 0.5 hours. Subsequently, the reaction mixture was poured into ice water and neutralized with 10% NaOH solution to pH 7. The resulting brown solution was filtered and the crude product was washed with distilled water and crystallized twice by ethanol to give a final orange solid product. The yield was 56% and the melting point was 134-136 °C. The product was analyzed by FT-IR (KBr, cm^-1 ) showing characteristic peaks: 2921, 2801, 2738, 1662, 1599, 1527, 1456, 1373. 1H NMR (300 MHz, CDCl3, ppm) data were as follows: δ 9.62 (1H, d, J=7.5 Hz), 7.48 (2H, d, J=7.5 Hz), 7.41 (1H, d, J=13.1 Hz), 6.71 (2H, d, J=7.5 Hz), 6.57 (1H, dd, J=7.5 Hz, 13.1 Hz), 3.08 (6H, s). Mass spectrometry (MS) analysis showed the molecular ion peak m/z: 176.2 (M+), consistent with the molecular formula C11H13NO.
3054-95-3
208 suppliers
$28.00/25g
586-77-6
438 suppliers
$5.00/10g

6203-18-5
293 suppliers
$6.00/250mg
Yield:6203-18-5 77%
Reaction Conditions:
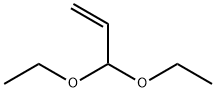
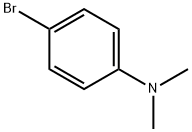
Stage #1: acrylaldehyde diethyl acetal;4-bromo-N,N-dimethylanilinewith potassium chloride;tetrabutylammonium acetate;potassium carbonate in N,N-dimethyl acetamide at 120; for 2.5 h;Heck reaction;
Stage #2: with hydrogenchloride in water;ethyl acetate at 20;chemospecific reaction;
References:
Alacid, Emilio;Najera, Carmen [European Journal of Organic Chemistry,2008,# 18,p. 3102 - 3106] Location in patent:experimental part
![2-Propen-1-ol, 3-[4-(dimethylamino)phenyl]-](/CAS/20210111/GIF/20850-05-9.gif)
20850-05-9
0 suppliers
inquiry

6203-18-5
293 suppliers
$6.00/250mg

586-77-6
438 suppliers
$5.00/10g
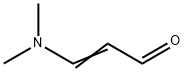
927-63-9
204 suppliers
$14.00/1g

6203-18-5
293 suppliers
$6.00/250mg

586-77-6
438 suppliers
$5.00/10g
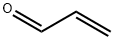
107-02-8
0 suppliers
$38.60/4s8501

6203-18-5
293 suppliers
$6.00/250mg
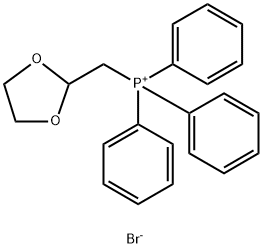
52509-14-5
232 suppliers
$5.00/5g

100-10-7
633 suppliers
$5.00/1g

6203-18-5
293 suppliers
$6.00/250mg