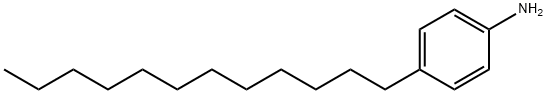
4-Dodecylaniline synthesis
- Product Name:4-Dodecylaniline
- CAS Number:104-42-7
- Molecular formula:C18H31N
- Molecular Weight:261.45
Yield:-
Reaction Conditions:
with aluminum (III) chloride;methyl tributylammonium chloride at 46 - 160; for 27.8 h;
Steps:
3
Example 3 Alkylation of Aniline Using Dodecenes, Aluminum Chloride and Methyltributylammonium Chloride An apparatus similar to that described in Example 1 was charged with aniline, dodecenes under nitrogen positive pressure, followed by aluminum chloride and then slow stirring started. There was an exotherm up to 46° C. upon stirring and then methyltributylammonium chloride was added. The reaction mixture was heated to 160° C. and maintained at this temperature for 27.8 hr. The reaction mixture was then allowed to cool to room temperature and diluted with 300 mL n-heptane-200 mL methylene chloride. The reaction mixture separated into a large volume upper phase and a dark red-orange lower phase. The upper layer was washed with 2×500 mL water, 500 mL aq. ammonia (400 mL water/100 mL conc. aq. ammonia) and then dried over anhydrous sodium sulfate. The sodium sulfate was removed by suction filtration and the filtrate stripped on a rotary evaporator in vacuo (5 mm final vacuum, 95° C. water bath). The residue was a reddish-orange oil, wt. 136.96 g. This was shown to be monododecylaniline by GC. IR analysis indicated the position of alkylation was mainly para.
References:
Hobbs, Steven J.;Madabusi, Venkatramanan K.;Wang, Jin-Yun;Stieber, Joseph F. US2007/135656, 2007, A1 Location in patent:Page/Page column 3
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

104-42-7
134 suppliers
$6.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

104-42-7
134 suppliers
$6.00/100mg
636-98-6
38 suppliers
$17.00/5g

104-42-7
134 suppliers
$6.00/100mg


